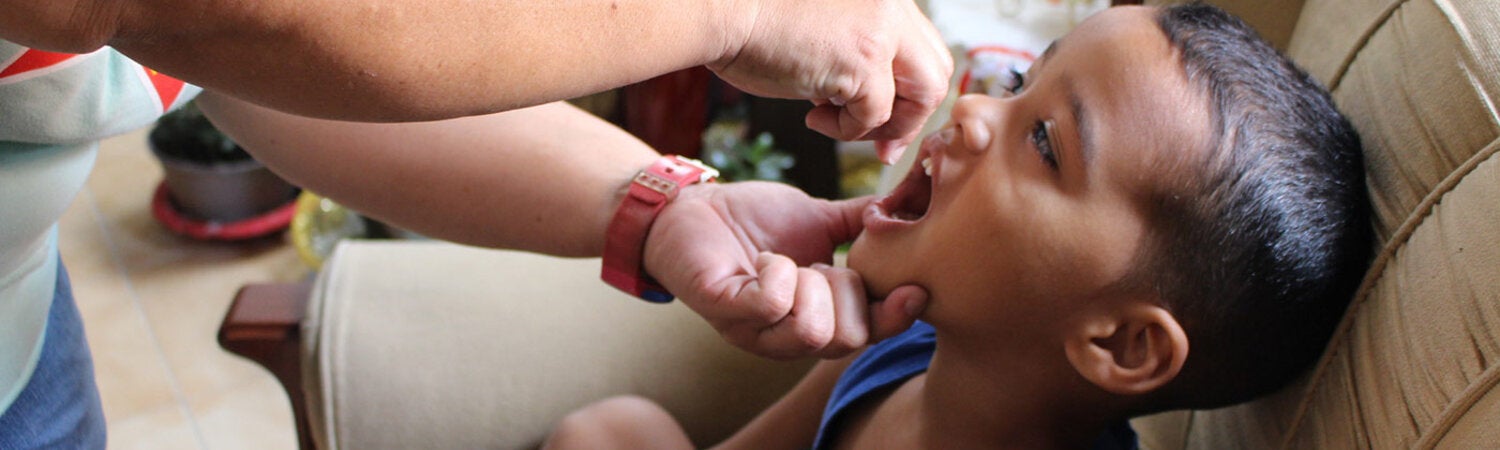
Poliomyelitis, commonly called polio, is a highly infectious disease, caused by the poliomyelitis virus. The vast majority of poliovirus infections do not produce symptoms, but 5 to 10 out of 100 people infected with polio may have some flu-like symptoms. In 1 in 200 cases, the virus destroys parts of the nervous system, causing permanent paralysis in the legs or arms. Although very rare, the virus can attack the parts of the brain that help you breathe, which can cause death.
Although the last confirmed case of poliomyelitis from wild poliovirus in the Region of the Americas occurred in 1991, the threat continues. Despite efforts to eradicate it, there continues to exist children with permanent paralysis due to this virus in some Asian countries. Because of the risk of importation, the main risk factor for children under 5 years of age to acquire this disease is low vaccination coverage.