SUBMENU
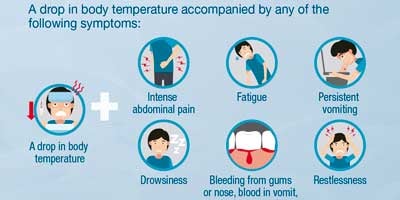
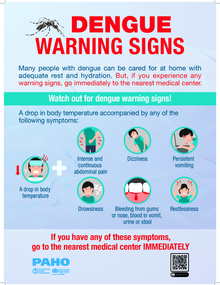
Dengue is transmitted by the bite of an infected mosquito. It is an illness that affects infants, young children, and adults, with symptoms ranging from mild fever to incapacitating high fever, with severe headache, pain behind the eyes, muscle and joint pain, and rash. The illness can evolve to severe dengue, characterized by shock, respiratory distress, severe bleeding, and/or serious organ impairment. The disease has a seasonal pattern: most cases in the southern hemisphere occur in the first half of the year, and most cases in the northern hemisphere in the second half. This pattern corresponds to the warmer, rainy months.
In the Americas, Aedes aegypti is the mosquito vector that is the main source of dengue transmission.
- Approximately 500 million people in the Americas are today at risk of dengue.
- Dengue incidence has increased in the Americas over the past four decades, from 1.5 million cumulative cases in the 1980s to 16,2 million in the decade 2010-2019.
- In 2023, the highest number of dengue cases was reported in the Region of the Americas, with a total of 4,565,911 cases, including 7,653 (0.17%) severe cases and 2,340 deaths (case fatality rate of 0.051%). This high transmission situation has extended into 2024, in which 673,267 cases of dengue were reported from epidemiological week (EW) 1 to EW 5, of which 700 were severe (0.1%) and 102 were fatal cases (case fatality rate 0.015%). This figure represents an increase of 157% compared to the same period in 2023 and 225% compared to the last 5-year average.
- The four dengue serotypes (DENV-1, DENV-2, DENV-3 and DENV-4) circulate throughout the Americas, and in some countries simultaneously.
- Following infection with one serotype, subsequent infection with a different serotype increases a person's risk of severe dengue and death.
- Ae. aegypti is the vector mosquito for dengue and is widely distributed in the Americas. Only Canada and continental Chile are free from dengue and its vector. Uruguay has no dengue cases, but it does have Ae. aegypti.
About Dengue
- It is transmitted by the bite of a mosquito infected with one of the four serotypes of dengue virus.
- It is a febrile illness that affects infants, children and adults. The infection may be asymptomatic, or it may present with symptoms ranging from a moderate fever to a disabling high fever, with severe headache, pain behind the eyes, muscle and joint pain, and rashes. The disease can evolve into severe dengue, characterized by shock, shortness of breath, severe bleeding and / or complications in the organs.
- There is no a specific medicine to treat dengue.
- The disease has a pattern according to the seasons: the majority of cases in the southern hemisphere occur in the first part of the year, and the majority of cases in the northern hemisphere occur in the second half.
- Dengue prevention and control must be intersectoral and involve the family and the community.
About Aedes aegypti
Aedes aegypti is the vector that presents the greatest risk of arbovirus transmission in the Americas and is present in almost all countries of the hemisphere (except Canada and continental Chile). It is a domestic mosquito (that lives in and near houses) that reproduces in any artificial or natural container that contains water.
The mosquito can complete its life cycle, from the egg to the adult, in 7-10 days; adult mosquitoes usually live 4 to 6 weeks. The female Aedes aegypti is responsible for the transmission of diseases because she needs human blood for the development of her eggs and for her metabolism. The male does not feed on blood.
The mosquito is most active early in the morning and at dusk, so these are the periods of greatest risk of bites. However, females, who need to continue feeding, will seek a source of blood at other times. The female Aedes aegypti feeds every 3-4 days; however, if they cannot draw enough blood, they continue feeding each moment they can.
Aedes aegypti prefers to lay its eggs in artificial containers that contain water (drums, barrels and tires, mainly) in and around homes, schools and workplaces. Aedes aegypti eggs can withstand dry environmental conditions for more than a year: in fact, this is one of the most important strategies that the species uses to survive and spread.
To eliminate mosquitoes, the following actions are recommended: avoid collecting water in open-air containers (pots, bottles or other containers that can collect water) so that they do not become breeding sites for mosquitoes; adequately cover water tanks and reservoirs to keep mosquitoes away; avoid accumulating garbage, throwing garbage in closed plastic bags.
- PAHO/WHO provides advice and technical support to prevent and control dengue. This work is carried out based on the Integrated Management Strategy for the Prevention and Control of Arboviral Diseases, which was adopted by PAHO/WHO Member States in 2016 (CD55.R6).
- In 2008, PAHO/WHO Member States established a Network of Dengue Laboratories of the Americas (RELDA) to strengthen technical capacities for dengue diagnosis. Currently, RELDA has been expanded to include chikungunya and Zika fever and comprises 40 laboratories in 35 countries in the Region.
- PAHO/WHO is supporting the development of an integrated epidemiological surveillance system model for dengue, chikungunya, and Zika. This model integrates epidemiological, clinical, laboratory and entomological surveillance to generate standardized and timely information for decision making. A technical document with guidelines for integrated epidemiological surveillance of arboviruses will be published in 2024. You can find the case definitions here.
- Under the concept of collaborative surveillance, PAHO/WHO develops and makes virtual collaboration spaces (ECV) available to countries. Through the ECVs, countries and PAHO collaborate in the real-time analysis of their epidemiological, clinical, laboratory, and entomological data, as well as the generation of automated reports and bulletins.
- PAHO/WHO promotes a clinical technical cooperation bundle in the countries to strengthen national capacities for clinical diagnosis and case management of dengue, chikungunya, and Zika in the Region. This bundle includes technical documents and clinical guidelines, virtual self-learning courses, a regional virtual classroom to train the trainers, and the formation of national networks of clinical experts in arboviral diseases.
- PAHO/WHO is working to strengthen regional and national capacity for the prevention and control of vectors. To achieve this, the organization has implemented the Plan of Action on Entomology and Vector Control 2018-2023. As part of this plan, various initiatives have been developed to improve entomological surveillance systems, monitor and manage resistance to insecticides used in public health, and train professionals in entomology through a virtual course on Surveillance and Control of Vectors of Public Health Importance. Additionally, PAHO/WHO is promoting the implementation of a new model of interventions for the control of Aedes aegypti and providing support to countries for the rational deployment of new technologies and approaches for vector control.