Washington, D.C., 25 April 2024 (PAHO) - The president of the Inter-American Development Bank (IDB), Ilan Goldfajn, and the director of the Pan-American Health Organization (PAHO), Jarbas Barbosa, signed an agreement to accelerate the digital transformation of health services, strengthen primary healthcare, and enhance pandemic preparedness across…
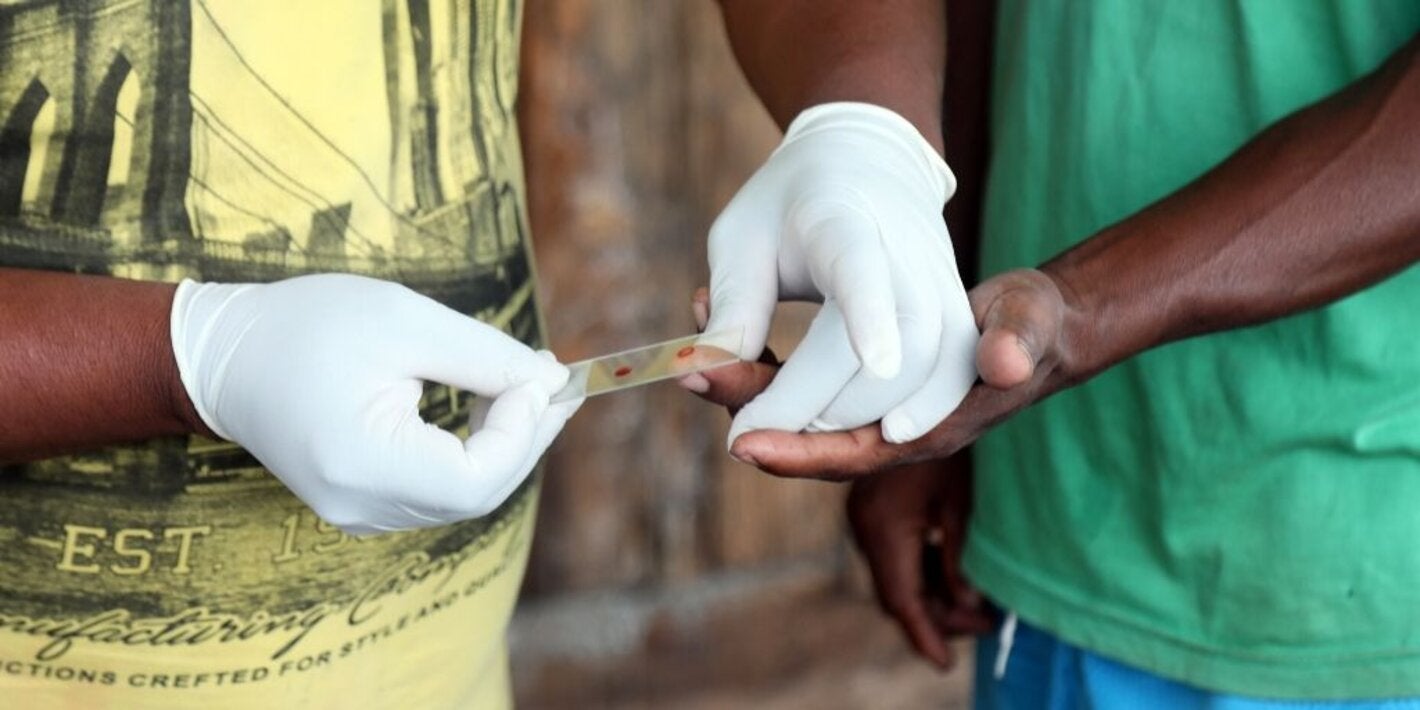
Washington D.C. 25 April 2024 (PAHO) – With countries of the Americas reporting around 480,000 cases of malaria in 2023, on World Malaria Day, the Pan American Health Organization (PAHO) has called on governments to step up efforts to tackle the disease, which disproportionately impacts indigenous communities, migrant and other vulnerable…
Washington D.C. 22 April 2024 (PAHO) – The 22nd edition of Vaccination Week in the Americas (VWA) 2024 was launched this week under the slogan “Engage now to protect your future #GetVax.” The launch ceremony was led by Pan American Health Organization (PAHO) Director, Dr. Jarbas Barbosa, as well as other high-level health authorities and regional…
Santiago de Chile, 19 April 2024 (PAHO) – While ensuring the technical quality of health care services is crucial, the Deputy Director of the Pan American Health Organization, Mary Lou Valdez, has called on countries to urgently “protect the basic conditions of hygiene, health, physical safety, and personal dignity,” in clinical settings.Speaking…
Washington D.C. 18 April 2024 (PAHO/WHO) – With 15 out of every 100 children in the Americas only partially protected against vaccine-preventable diseases, the Pan American Health Organization (PAHO) Director, Dr. Jarbas Barbosa, has urged countries of the region to continue efforts to recover routine vaccination coverage. “Historically, our…
Washington, D.C., April 16, 2024. In the context of the 22nd Vaccination Week in the Americas, celebrated from 20-27 April, the Pan American Health Organization (PAHO) is sharing more than 15 innovative experiences that countries in the Region have implemented to promote vaccination in their communities. The slogan of the week this year is…
Brasilia, April 15, 2024 - The Pan American Health Organization (PAHO) recently presented a series of recommendations at G20 events in Brazil to strengthen equity in access to digital health, expand innovation and production of health technologies, tackle antimicrobial resistance, increase health investment and ensure greater retention of health…
Ottawa, Canada, 11 April 2024 (PAHO) – During an official visit to Canada this week, Pan American Health Organization (PAHO) Director, Dr. Jarbas Barbosa, met with the country’s Minister of Health, Mark Holland, as well as other high-level health officials, to discuss ongoing collaboration to enhance quality of life and health in the Americas.At…
Geneva, 10 April, 2024 — According to the World Health Organization (WHO) 2024 Global Hepatitis Report, the number of lives lost due to viral hepatitis is increasing. The disease is the second leading infectious cause of death globally -- with 1.3 million deaths per year, the same as tuberculosis, a top infectious killer.The report, released at…