Immunization is the process whereby a person is made resistant to a disease, typically by the administration of a vaccine. Vaccines stimulate the body’s own immune system to protect the person against subsequent infection or disease. Immunization prevents diseases, disabilities, and deaths from vaccine-preventable diseases (VPDs), such as cervical cancer, poliomyelitis, measles, rubella, paroditis, diphtheria, tetanus, pertussis, hepatitis A and B, bacterial pneumonias, rotavirus diarrheal diseases and bacterial meningitis.
The Special Program Comprehensive Immunization (CIM) seeks to promote and coordinate technical cooperation and partnerships to support Member States' efforts to sustainably and equitably reduce morbidity and mortality from vaccine-preventable diseases (VDPDs) through control and elimination strategies to improve the quality of life and life expectancy of the peoples of the Americas.
For over 40 years, the success of the Expanded Program on Immunization (EPI) has made the Region of the Americas a global leader in the elimination and control of vaccine-preventable diseases (VPDs), such as smallpox, polio, rubella, congenital rubella syndrome, measles, and neonatal tetanus. Since the creation of the EPI in 1977, countries have moved from using six vaccines in their national vaccination schemes, to an average of more than 16 vaccines, which represents greater protection for the population.
Within the framework of the resolution "Reinvigorating immunization as a public good for universal health" approved in 2021 by PAHO's governing bodies, CIM seeks to revitalize immunization programs in Member States by implementing innovative approaches and best practices through six lines of action:
- Strengthen governance, leadership and financing of immunization programs.
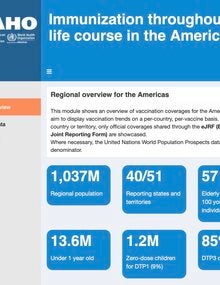
- Improve immunization coverage monitoring and surveillance by incorporating digital intelligence strategies into routine analysis
- Strengthen the integration of immunization programs into the primary health care system toward universal health
- Develop innovative and strategic communication approaches to build social awareness and trust in vaccines and increase access to services
- Strengthening human resource capacities for immunization programs
- Using scientific evidence to guide decision making and program delivery
Immunization Toolkit
This toolkit has been created and it is updated as a resource to support managers of the Expanded Programs on Immunization (EPI) in the Region, as well as their teams, in all aspects of the planning, monitoring, reporting and evaluation of immunization programs.
This toolkit also serves as a reference library for Technical Advisory Group (TAG) for Immunization recommendations, policy resolutions from PAHO’s Governing Bodies on immunization-related topics, pertinent guidelines and field guides, and key publications.