:: Background
Influenza infections and their complications represent a significant burden of morbidity and mortality in the region of the Americas. It is estimated that around 79,057 deaths from influenza, varying between 48,880 and 160,270, occur annually in the Americas, 81% of the deaths being adults ≥65 years (data from 35 countries). The findings available for Latin America suggest that the disease most severely affects children <5 years and adults ≥60 years with pre-existing conditions. Influenza vaccines are one of the most effective measures to prevent severe influenza illness and its complications. The currently used vaccination contains antigens against three strains of seasonal influenza viruses (A / H1N1, A / H3N2 and B (Yamagata or Victoria lineage). Given the frequent genetic changes that characterize the virus, the components of the vaccine need to be updated annually, taking into account counts the differences between the epidemics of the Southern Hemisphere and the Northern Hemisphere The vaccine composition selection committee at the World Health Organization (WHO) reviews virological and epidemiological information at the global level and recommends the strains that are predicted to circulate the next season. The effectiveness of the vaccine depends, in addition to the age and health status of the vaccinated, on the concordance between the vaccine strains and the circulating strains. Due to the heterogeneity of the aforementioned influenza viruses, genetic changes may occur even during the same season, decreasing the effectiveness of the vaccine. For these reasons, it is necessary to know the performance of the vaccine annually and have evidence for an adequate decision-making in public health. Determining a low vaccine effectiveness at the beginning of an epidemic may be useful to guide the implementation of other complementary measures for the prevention and control of the disease.
To generate systematic evidence on vaccine effectiveness to guide interventions and evaluate the impact of existing vaccination programs, the Pan American Health Organization (PAHO), and the Influenza Division of the US Centers for Disease Control and Prevention (CDC) ) explored in 2012 with some countries in the region, the possibility of evaluating the effectiveness of the influenza vaccine in a regional, multicenter project based on the existing influenza surveillance platform.
In this context, a pilot phase was carried out in 2012 in 4 Central American countries with the support of the local offices of PAHO, and of the Central American office of the network for training in field epidemiology (TEPHINET) for the development of activities. field. In March 2013, the experiences and lessons learned from the pilot were shared with teams from the ministries of health of 8 more countries in the region and the official creation of a Network for the Evaluation of Influenza Vaccines in Latin America and the Caribbean was proposed (REVELAC -i) The implementation phase of the vaccine effectiveness evaluation had the participation of Argentina, Brazil, Chile, Colombia, Costa Rica, El Salvador, Honduras, Panama and Paraguay during the 2013 influenza season.
To date, 15 countries have joined the REVELAC-i network for which the following objectives have been established:
- Generate mechanisms to share experiences, lessons learned and common methods between countries and research centers on the effectiveness of the influenza vaccine, as well as to know the impact of vaccination on morbidity and mortality due to influenza.
- Continue the integration of data from epidemiological and virological surveillance and immunization programs to generate evidence for the prevention and control of influenza.
Consequently, it is expected that the data provided by the evaluation of the effectiveness of the vaccine will serve the same users of the surveillance system to complete the sentinel surveillance information and support evidence-based decision making. Likewise, it is recommended that this evaluation be integrated as another objective of the SARI surveillance system itself or that it be considered as secondary data analysis of surveillance. The results of the effectiveness analysis may contribute to complementary analyzes necessary for vaccination programs, such as measuring their impact, or the costs avoided by vaccination.
:: Objectives
- Form a network of institutions generating evidence on influenza vaccines in Latin America and the Caribbean, capable of providing and sharing information quickly and reliably on the effectiveness of the influenza vaccine annually and the impact of this prevention strategy, among others.
- Provide information on the effectiveness of the seasonal influenza vaccine in a systematic and timely manner to decision makers in Member States and to public health experts, health workers, and the general population.
In 2013, Argentina, Brazil, Chile, Colombia, Costa Rica, Cuba, El Salvador, Honduras, Nicaragua, Panama, Paraguay and Uruguay joined the network.
During the 2013 influenza season, 9 countries participated in the multicenter vaccine effectiveness evaluation (Argentina, Brazil, Chile, Colombia, Costa Rica, El Salvador, Honduras, Panama, and Paraguay).
In 2014, Cuba and Mexico participated in the evaluation of the influenza vaccine.
In 2015 (Peru) and in 2016 (Uruguay), they conducted pilot studies.
In 2020-2022, six countries: Chile, Costa Rica, Ecuador, Guatemala, Paraguay and Uruguay contributed their data to estimate the effectiveness of COVID-19 and influenza vaccines.
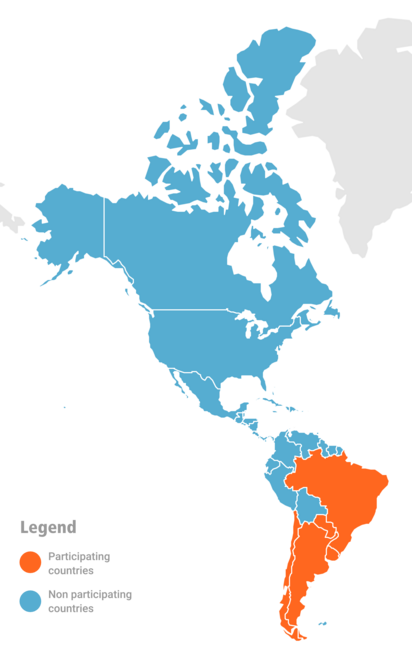
In 2023, 5 countries in the southern hemisphere participated in the evaluation of influenza vaccine effectiveness: Argentina, Brazil, Chile, Paraguay and Uruguay.
:: Acta
Declaración técnica de la ciudad de Antigua para la creación de una red para evaluación de efectividad de la vacuna contra influenza en Latino America y el Caribe Antigua, Guatemala, 27 de febrero de 2013
Antigua, Guatemala, 27 de febrero de 2013 En la reunión técnica de Influenza realizada en la ciudad de Antigua, Guatemala del 25 al 27 de febrero, participaron de América Latina y el Caribe, Centros de Investigación y Agencias de Cooperación Técnica y
Reconocieron que:
La Influenza constituye un serio problema de salud pública una alta carga de enfermedad y muerte en la Región, especialmente en los grupos de riesgo.
La mayoría de los países han hecho grandes avances en la introducción de la vacuna contra influenza estacional.
Los países de la Región han fortalecido los sistemas de vigilancia epidemiológica, de laboratorio y existen unidades centinela para la vigilancia de los virus respiratorios.
Costa Rica, El Salvador, Hondura y Panamá, participaron del primer proyecto piloto multicéntrico en países en vías de desarrollo para la evaluación de la efectividad de la vacuna de influenza.
Es necesario contar con evidencia para la adecuada toma de decisiones en salud pública, teniendo en cuenta que la efectividad de la vacuna de influenza varía cada año dependiente de la edad, grupo de riesgo y la concordancia de las cepas de la vacuna con las cepas circulantes, por lo cual es importante conocer el desempeño de la vacuna de manera sistemática.
Para obtener el archivo completo, lo puede descargar en este enlace.
- Entry requirements
- Operating requirements
- Permanence requirements
- Different status of participation in the network
Regional Coordination Team
- Francisco Nogareda, CIM, OPS, Washington D.C.
- Paula Couto, Vigilancia de influenza, OPS, Washington D.C.
- Jorge Jara, CIM, OPS, Washington D.C.
- Martha Velandia, CIM, OPS, Washington D.C.
- Marc Rondy, Vigilancia de inluenza, OPS, Washington D.C.
- Antonio Méndez, encargado del gestor de datos. CIM, OPS
- Annette Regan, estadística, CIM, OPS
- Eduardo Azziz-Baumgartner, División de Influenza, CDC, Atlanta.
- Ashley Fowlkes, División de Influenza, CDC, Atlanta.
National Teams
Argentina
- Carlos Giovacchini, Dirección Nacional de Epidemiología e Información Estratégica. Ministerio de Salud de la Nación.
- Carla Voto, Dirección Nacional de Epidemiología e Información Estratégica. Ministerio de Salud de la Nación.
- Teresa Strella, Dirección Nacional de Epidemiología e Información Estratégica. Ministerio de Salud de la Nación.
- Florencia Bruggesser, Control de Enfermedades Inmunoprevenibles. Ministerio de Salud de la Nación.
- Nathalia Katz. Control de Enfermedades Inmunoprevenibles. Ministerio de Salud de la Nación.
- Analia Rearte. Dirección Nacional de Epidemiología e Información Estratégica. Ministerio de Salud de la Nación.
- Wilmer Marquiño. OPS, Argentina
- Agustina Iovane. OPS, Argentina
Brazil
National Team Brazil:
- Walquiria Aparecida Ferreira da Almeida, Ministerio de Salud, Brasil
- Daiana Araújo da Silva, Ministerio de Salud, Brasil
- Greice Madeleine Ikeda do Carmo, Ministerio de Salud, Brasil
- Francisco José de Paula Júnior, Ministerio de Salud, Brasil
- Felipe Cotrim de Carvalho, Ministerio de Salud, Brasil
- Hellen Kássia Rezende Silva, Ministerio de Salud, Brasil
- Sirlene de Fátima Pereira, Ministerio de Salud, Brasil
- Miriam Teresinha Furlam Prando Livorati, Ministerio de Salud, Brasil
- Marilda Agudo Mendonça Teixeira de Siqueira, Oswaldo Cruz Foundation (Fiocruz) – Centro Nacional de Influenza (NIC), asociado al Ministerio de Salud, Brasil
Federated Units in Brazil:
Telma Carvalhanas and Pamella Cristina de Carvalho Lucas (São Paulo); Acácia Maria Lourenco Francisco Nasr y Ana Carolina Geffer Dalla Vecchia Fiorentin (Paraná); Adriana Pimentel Veras y Bianca Brandão Almeida (Para); Gilmar Jose Coelho Rodrigues y Bruna Dias Tourinho (Minas Gerais); Lívia de Mello Almeida Maziero y Naira Rocha Chaves (Mato Grosso do Sul); Fernanda Carolina Rodrigues Vieira y Talitha Emanuelle Barbosa Galdino de Lira Santos (Paraíba); Cleidiane Santos Rodrigues de Carvalho y Rosana Aparecida Campos Coelho (Distrito Federal); Aline Anne Ferreira de Deus (Bahia); Letícia Garay Martins y Carolina Nunes Port (Rio Grande do Sul); Tatiana Luciano Sardeiro y Mônica Aparecida Henrique de Oliveira (Goiás); Alexsandro Melo y Inaiah Ordones (Amazonas);
PAHO Brazil:
- Lely Guzman
- Patrícia Marques Ferreira
- Seiarameri Lana Viola Oliveira
Chile
- María Fernanda Olivares, Vigilancia centinela de influenza, Departamento de Epidemiología Ministerio de la Salud.
- Natalia Vergara, Departamento de Epidemiología Ministerio de la Salud, Departamento de Estadísticas e información en Salud.
- Marcela Avendaño, Programa nacional de inmunizaciones, Ministerio de la Salud.
- Rodrigo Fasce, laboratorio de referencia, Instituto de Salud Pública.
- Solange Santillana. OPS, Chile
- Claudio Canales. OPS, Chile
Paraguay
- Marta Von Horoch, Programa Ampliado de Inmunizaciones, Ministerio de Salud y Bienestar Social.
- Silvia Battaglia, Programa Ampliado de Inmunizaciones. Ministerio de Salud y Bienestar Social.
- Elena Penayo, Vigilancia Epidemiológica. Ministerio de Salud y Bienestar Social.
- Chavely Dominguez, Vigilancia Epidemiológica. Ministerio de Salud y Bienestar
- Cynthia Vázquez, Laboratorio Central de Salud Pública. Ministerio de Salud y Bienestar Social.
- M José Ortega, Laboratorio Central de Salud Pública. Ministerio de Salud y Bienestar Social.
- Neris Villalobos, OPS, Paraguay
- Romeo Montoya, OPS, Paraguay
Uruguay
- Natalia Goñi. Departamento de Laboratorios de Salud Pública, Ministerio de Salud Pública
- Hector Chiparelli. Departamento de Laboratorios de Salud Pública, Ministerio de Salud Pública
- Karina Guiot. Departamento de Vigilancia de la Salud. Ministerio de Salud Pública
- Miguel Alegretti. Departamento de Vigilancia de la Salud. Ministerio de Salud Pública
- Adriana Alfonso. Departamento de Vigilancia de la Salud. Ministerio de Salud Pública
- Grisel Rodríguez, OPS, Uruguay
- Lucia Alonso, OPS, Uruguay
Projects/Associated Agencies
- SARInet, OPS
- CDC/Influenza Division
- CDC/CORVD
- CLAP, OPS
- EUROSAVE, WHO
- VEBIS, ECDC
I-MOVE in Europe (Influenza – Monitoring Vaccine Effectiveness)
Project aims at measuring influenza vaccine effectiveness in Europe. The project started in 2007. In the first four seasons of I-MOVE (2008-9 to 2011-12) the European Centre for Disease Prevention and Control (ECDC) and countries conducting studies co-funded the project. In 2012-13 I-MOVE is co-funded by the countries conducting studies and by EpiConcept.
SARInet – The severe acute respiratory infections network in the Americas
Through this platform you will be able to:
- Share and discuss experiences implementing SARI surveillance
- Discuss surveillance strategies and challenges with experts
- Access scientific peer-reviewed public health publications
- Collaborate in regional projects to estimate burden of viral respiratory diseases
- Access up-to-date surveillance training materials
- Access up-to-date surveillance and laboratory guidelines