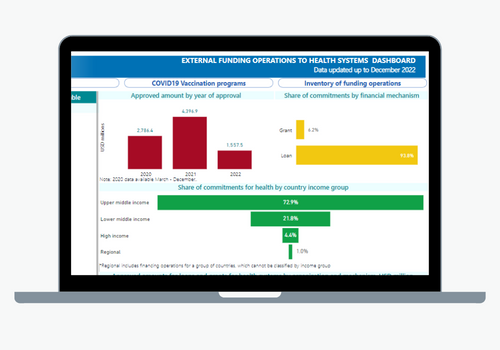
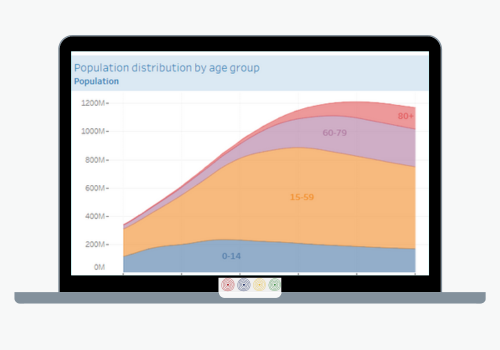
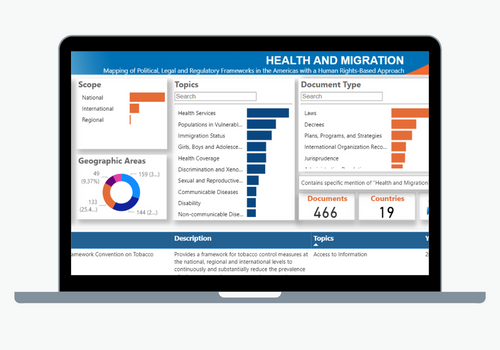
The primary objective of the Department of Health Systems and Services is to strengthen health systems based on Primary Health Care, support the transformation of national health systems to improve equity and resilience, strengthen Primary Health Care (PHC), and address the relevant issues related to human resources as health systems move towards the achievement of Universal Health. Its work encompasses the following areas: integrated services delivery, strengthening of health systems governance and stewardship; developing efficient and equitable financial mechanisms to ensure adequate public funding of the health system; human resources for health, healthy life course; women’s, maternal, neonatal and reproductive health; and the Virtual Campus of Public Health.
Alliance for Primary Health Care in the Americas
The Alliance for Primary Health Care in the Americas was established in December of 2023 between the Pan American Health Organization, the Inter-American Development Bank and the World Bank. It aims to bolster investment, innovation, and implementation of policies and initiatives to transform health systems in the Region, with a strong emphasis on primary health care.
The Decade of Healthy Aging in the Americas (2021-2030)
The Decade of Healthy Ageing 2021-2030, declared by the United Nations General Assembly in December 2020, is the main strategy to build a society for all ages. PAHO leads the concerted agenda of the Decade of Healthy Aging in the Americas 2021-2030.
Zero Preventable Maternal Deaths
PAHO calls on countries to promote immediate action at the national and subnational level, with recommendations to face the main structural challenges of maternal health with the aim of accelerating the reduction of maternal mortality.
Virtual Campus for Public Health
The Virtual Campus for Public Health (VCPH) is PAHO's educational platform. Its purpose is to help develop the skills and competencies of health workers by transforming public health services and practices in the Region of the Americas.
Dr Barbosa (PAHO Director) — WHO Traditional Medicine Global Summit
Traditional Medicine Region of the Americas
Building health throughout the life course
Dr Jarbas Barbosa, Director of PAHO - Health for All 2023
Decade of Healthy Aging in the Americas
Virtual Campus for Public Health PAHO/WHO
VCPH Educational Approach