In Chile, pharmacies are health centers where health care is performed. Pharmacies cooperate with each other to ensure rational drug use in health care.
As of December 2019, there were 3,747 authorized pharmacies in Chile, of which 1,757 were concentrated in the Santiago Metropolitan Region, and 1,990 in the other 15 regions of the country.
During each enforcement procedure, a checklist is applied to determine whether regulations are met. Non-compliance can lead to the application of health measures aimed at protecting the population.
The National Drug Agency (Departamento Agencia Nacional de Medicamentos, ANAMED) of the Public Health Institute of Chile (Instituto de Salud Pública, ISP), is responsible for the enforcement of pharmaceutical drugs and cosmetics manufactured locally or imported for marketing in the country, ensuring their quality, safety, and efficacy.(1)
ANAMED grants health authorizations, registers pharmaceutical drugs and cosmetics, and actively audits and monitors these products.(1)
The enforcement procedures can be carried out during field visits or by analyzing documents. Inspections are generally classified as planned visits (according to annual schedules) or unplanned visits (according to demand).(2)
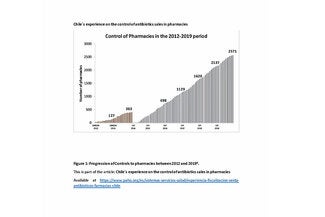
With the entry into force of the Pharmaceuticals Act (Ley de Fármacos I) (3) in 2014, pharmacies became subject to ISP enforcements. Previously, this was done by the Chilean Ministry of Health (MINSAL) through the regional ministerial secretariats (SEREMI). Currently, the ISP conducts enforcement actions in the Metropolitan Region of Santiago (Figure 1) and SEREMI covers the other regions of the country.
.
As of December 2019, there were 3,747 authorized pharmacies in Chile, of which 1,757 (47%) were concentrated in the Santiago Metropolitan Region and 1,990 were in the other 15 regions. (4)
A checklist is used during the enforcement procedures in order to determine whether specific regulations are met. Non-compliance can lead to the implementation of health measures aimed at protecting the population. (5)
In Chile, pharmacies are health centers where health care is performed. Pharmacies cooperate with each other to ensure the rational use of medicines in health care. Pharmacies must employ a pharmaceutical chemist, have a supply of essential medicines, guide patients on rational use of medicines, and contribute to pharmacovigilance efforts.
The enforcement tool developed by ANAMED does not currently include specific items to directly audit antibiotics sold in pharmacies. However, the evaluation of compliance with conditions of sale could be a starting point for controlling the sale of antibiotics.
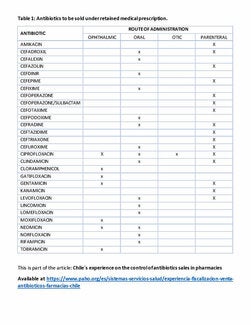
In Chile there are currently 29 antibiotics that must be sold with a prescription that is filed in the pharmacy. This involves filling out pharmaceutical forms on parenteral, oral, ophthalmic, and ophthalmic use (see Table 1). (6) In the first phase, the enforcement procedures could focus on these medicines, as it is possible to objectively compare the number of filed prescriptions with the number of sales, allowing for assessment of compliance with the conditions of sale for antibiotics. The second phase could expand to other antimicrobials, such as antivirals, antifungals, and antiparasitic drugs.
This specific enforcement plan should be included in the general plan for inspections. An additional checklist item will be evaluated, containing the following information to be verified:
1. Stock of antibiotics subject to sale with a filed prescription.
2. Existence of filed prescriptions for this type of antibiotic.
3. The sales record matches the number of filed prescriptions.
When the results of the audits have been obtained, a baseline will be established for the performance of pharmacies in terms of dispensing antibiotics. If necessary, these results will guide the future assessment of health measures to improve the rational and safe use of antimicrobials.
Disclaimer
Authors hold sole responsibility for the views expressed in their texts, which may not necessarily reflect the opinion or policy of the Pan American Health Organization. The mention of specific companies or certain manufacturers' products does not imply that they are endorsed or recommended in preference to other ones of a similar nature.
Note: Article subscribed by María Francisca Aldunate González, Chief of the Medicine Information Section, Sub-Department of Pharmacovigilance, National Agency of Medicines Department of the Public Health Institute, to PAHO/WHO for the Bulletin on Rational Use for of antimicrobials for the containment of the resistance.
1. Instituto de Salud Pública. Quiénes Somos. [online]. Available from: http://www.ispch.cl/anamed_/quienessomos [cited 2019 Aug 8].
2. Instituto de Salud Pública. Formularios e instructivos de solicitudes Farmacias [online]. Available from: http://www.ispch.cl/anamed_/quienessomos [cited 2019 Aug 8].
3. Ministerio de Salud. LEY Nº 20.724 Modifica Código Sanitario en materia de regulación de farmacias y medicamentos. Published 2014 Feb 14.
4. Instituto de Salud Pública. Base de datos Subdepartemanto Fiscalizaciones. Cited 2020 July.
5. Instituto de Salud Pública. Actividades de Inspección, Fiscalización y Control. [online]. Available from: http://www.ispch.cl/anamed_/establecimientos_sanitarios/fisc_control [cited 2019 Aug 8 ].
6. Instituto de Salud Pública. Base de datos GICONA. Cited 2019 Aug.
More information