PAHO Ethics Review Committee (PAHOERC)
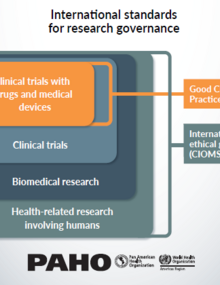
PAHOERC ensures that all research carried out with PAHO support, whether technical or financial, follows international standards for research with human participants. All research with human participants must also receive approval from an independent Ethics Review Committee (ERC) in the country where the study will take place. PAHOERC is the entity responsible for determining whether a proposal meets the definition of "research involving human participants." For more information, consult the PAHOERC Standard Operating Procedures (SOP).
PAHOERC is made up of a group of professionals from different disciplines, observers from key departments, and a Secretariat that is part of the Regional Program on Bioethics, within the Science and Knowledge for Impact Unit of the Department of Evidence and Intelligence for Action in Health (EIH/SK). Current PAHOERC members and observers are listed here.
PAHOERC 2025
:: Members
- María Paz Ade (President), Advisor, Malaria Diagnostics and Supply Management; Neglected, Tropical and Vector Borne Diseases
- Mauricio Beltrán Durán, Advisor, Blood Transfusion and Organ Transplants
- Regina Campa Sole, Advisor, Partnerships/External Relations, Partnerships and Resource Mobilization
- Carolina Chávez Cortes, (External), Former International PAHO Consultant, Noncommunicable Diseases, Violence and Injury Prevention
- Margherita Ghiselli, Advisor, Immunizations, Special Program, Comprehensive Immunization
- Aleida Domingo Gisbert, Administrative Assistant, Strategic Fund for Public Health Supplies
- Lionel Gresh (Vice President), International PAHO Consultant, Viral Diseases
- Maristela Monteiro, (External), Former PAHO Senior Advisor, Alcohol and Substance Abuse
- Bernardo Nuche Berenguer, International PAHO Consultant, HIV, Hepatitis, Tuberculosis, and Sexually Transmitted Infections
- Pedro Ordúñez, Advisor, Chronic Diseases
- Hernán Rodriguez, Advisor, Health Systems and Services, Peru
- Carla Saenz (Secretary), Regional Bioethics Advisor
- Omar Sued, Advisor, HIV/STI Care and Treatment, HIV, Hepatitis, Tuberculosis, and Sexually Transmitted Infections
- Sergio Surugi de Siqueira, (External), Professor (Universidade Federal do Paraná); former member of Brazil’s National Research Ethics Committee (CONEP)
:: Observers
- Gabriela Gergely, Ethics Office
- Christina Marsigli, Department of External Relations, Partnerships and Resource Mobilization
- Shirley Quesada, Department of Procurement and Supply Management
- Nicole Wynter, Department of Planning, Budget and Evaluation
- Pamela Zúñiga, Office of the Legal Counsel
:: Secretariat
- Carla Saenz (Secretary), Regional Bioethics Advisor
- Magalys Quintana (Secretariat Support), Ethics Review Specialist
Submitting a proposal to PAHOERC
Proposals for review or consultation (see FAQs below) must be submitted through ProEthos, PAHOERC's portal for ethics review. After creating an account in ProEthos, follow the instructions in the platform to submit the proposal. Questions can be directed to PAHOERC@paho.org.
SEND PROPOSAL
PAHOERC INTRANET ACCESS
PAHOERC VIDEOS
Information Session
What needs ethics review during the pandemic?
Videos on Research Ethics
When does health research need ethics review?
What do Research Ethics Committees review?
External Resources
- Qualitative research and evaluation methods: integrating theory and practice, 4th Edition (Sage Publishing)
- Qualitative research methods for the social sciences 9th Edition (Amazon)
- Online courses on methods in social sciences (NEW)