SUBMENU
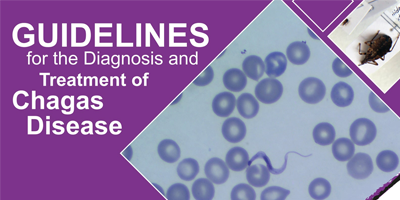
Chagas disease is a parasitic, systemic, and chronic disease caused by the protozoan Trypanosoma cruzi, with risk factors strongly link to low socioeconomic factors. Chagas disease is considered a neglected tropical disease. It is endemic in 21 countries in the Americas, although the migration of infected people can transport the disease to non-endemic countries of America and the world.
T. cruzi parasites are mainly transmitted to human by the infected feces of blood-sucking triatomine bugs, known as the "kissing bug". T. cruzi can infect several species of the triatomine bug, the majority of which are found in the Americas. A person becomes exposed when the infected insect deposits its feces in the person's skin when he or she is sleeping during the night. The person will scratch the infected area, unintentionally introducing the insect's feces in in the wounds of the skin, the eyes, or the mouth. Other modes of transmission are through blood transfusion, congenital, and organ transplants. Although mortality has significantly declined, the disease can cause irreversible and chronic consequences on the heart, digestive system, and nervous system.
- Chagas disease can be treated with Benznidazole and also Nifurtimox. Both medicines are almost 100% effective in curing the disease if given soon after infection at the onset of the acute phase.
- Chagas disease is endemic in 21 countries in the Americas, and affects approximately 6 million people.
- In the Americas, Chagas disease show an annual incidence of 30,000 new cases average, 12,000 deaths per year, and approximately 9,000 newborns become infected during gestation.
- It is estimated that around 70 million people in the Americas live in areas of exposure and are at risk of contracting this disease.
- Since the early 1990s, the countries affected by Chagas disease have organized to provide a public health response in conjunction with the PAHO/WHO, generating a successful horizontal technical cooperation scheme between countries, through Sub-regional Initiatives of Prevention and Control of Chagas Disease (Southern Cone, Central America-Mexico, Andean countries, and Amazonian countries).
- These countries have managed great achievements in controlling vector-borne transmission of T. cruzi including the interruption of vector-borne transmission in 17 affected countries and the elimination of certain species of vectors, implementation of universal screening of blood donors for Chagas disease in 21 endemic countries, increased coverage, and capacities of diagnosis and treatment of congenital cases of Chagas, expanded coverage of diagnosis and access to treatment, and clinical care of people infected and sick.
- El Salvador, Costa Rica, Honduras, Nicaragua, Guatemala, and Mexico reached the elimination of Rhodnius prolixus as the main vector between 2009 and 2010, respectively. South America reached the elimination by T. infestans vector, in Brazil (Sao Paulo) and Uruguay, in 2012 and 2014, respectively.
- Through PAHO/WHO, the countries receive between 3,000 and 4,000 Nifurtimox treatments each year. Benznidazole, which is now produced in Argentina and Brazil, can be purchased through the PAHO/WHO Strategic Fund.
- The World Health Assembly 2010 resolution WHA 63.20 and PAHO/WHO 2010 resolution CD50.R17, establish and implement the current Strategy and Plan of Action for Chagas disease prevention, control, and care.
- PAHO/WHO 2009 resolution CD49.R19 and 2016 resolution CD55.R9 of Neglected Infectious Diseases, provide a frame of reference on controlling and eliminating Chagas disease as a public health problem.