The Pan American Network for Drug Regulatory Harmonization (PANDRH) is an initiative of the national regulatory authorities within the Region, and PAHO, that supports the processes of pharmaceutical regulatory harmonization in the Americas, within the framework of national and sub-regional health policies and recognizing pre-existing asymmetries.
Objectives, structure, resolution, and statutes
The Pan American Network for Drug Regulatory Harmonization (PANDRH) started in 1999, and its components are the Pan American Conference on Drug Regulatory Harmonization, the Steering Committee, the Secretariat, and the technical structures needed to implement projects in the agreed strategic areas.
PANDRH's general objectives are to:
- Strengthen the regulatory functions and systems of the countries of the Region, promoting cooperation and sharing among countries, with the Pan American Health Organization (PAHO), and with other regional and international organizations, civil society, industry associations, and academia.
- Develop, approve, and implement common proposals (projects, joint activities, technical documents, guidelines, work plans, etc.) for the regulation of health technologies, considering international guidelines and standards for regulatory convergence.
- Develop core competencies aimed at supporting and strengthening good regulatory practices and regulatory science in the Member States with the goal of achieving regulatory convergence in the Region. Encourage the NRAs of the Region to develop and maintain well-structured organizations to achieve effective regulatory functions as an essential part of health systems, in accordance.
- The Components of PANDRH are The Pan American Conference on Drug Regulatory Harmonization (PANDRH), the Steering Committee (SC), the Technical Working Groups (WGs) in the areas considered as a priority by the Conference, and the Secretariat.
Consult the Statutes of the Pan American Network for Drug Regulatory Harmonization
PANDRH´s Members
The components of the Pan American Network for Drug Regulatory Harmonization (PANDRH) are the Pan American Conference on Drug Regulatory Harmonization, the Steering Committee, the Secretariat, and the technical structures needed to implement projects in the agreed strategic areas.
These conferences constitute a continental forum that deals with drug regulatory harmonization. It includes the presence of all the region's drug regulatory authorities, as well as representation of organisms for economic integration such as CARICOM, MERCOSUR, NAFTA, Latin American Association for Integration (ALADI) and the Andean Community; Academics; representation of regional professional association; and other groups interested from all the continent sub-regions.
The conference constitutes a way to disseminate the decisions on drug regulatory harmonization of global initiatives, such as the International Conference of Drug Regulatory Authorities (ICDRA) and the International Conference on Harmonization (ICH). The PANDRH has become a member of the Global Cooperation Group of the ICH.
The Conference also facilitates the integration of the countries from the continent that do not belong to the sub-region blocks, as is the case of Cuba, the Dominican Republic, and Chile. Its main objective is to support the harmonization processes through the analysis of specific aspects and the adoption of recommendations on priority subjects and harmonized guidelines proposed by the working groups established by the conference itself. Conclusion and recommendations Conference are expected to use by the economic integration groups in its harmonization processes and by all drug regulatory authorities in the countries of the region.
The Steering Committee is the decision-making body for the strategic and operational management of the Network, providing guidance for progress on projects and activities, and making recommendations for evaluation and discussion at the Conference.
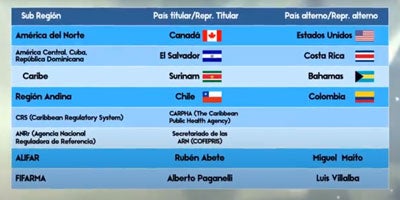
The Steering Committee is be composed of the Secretariat and of Members and their corresponding Alternates, officially designated to represent each sub-Region of the Region of the Americas, as follows: North America, Central America + Cuba + the Dominican Republic, the Caribbean, the Andean Region, and the Southern Cone.
The Steering Committee will reserve a private space for decision-making by the representatives of the health authorities or Regional regulatory systems/frameworks appointed in accordance with the following:
- North America: United States, Canada, and Mexico
- Central America + Cuba + Dominican Republic: Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua, Panamá, Cuba, Dominican Republic
- Caribbean: Antigua and Barbuda, Bahamas, Barbados, Belize, Dominica, Grenada, Guyana, Haiti, Jamaica, Montserrat, St Kitts and Nevis, Saint Lucia, St Vincent and the Grenadines, Suriname, Trinidad, and Tobago
- Andean Region: Bolivia (Plurinational State of), Chile, Colombia, Ecuador, Peru, Venezuela (the Bolivarian Republic of)
- Southern Cone: Argentina, Brazil, Paraguay, Uruguay
Steering Committee Members will be appointed for a four-year term, ensuring rotation among the countries in each sub-Region and communicating officially to the Secretariat. Rotation will occur at the Pan American Conferences were, to ensure continuity of work, up to three of the five members and alternates should be changed, according to their seniority.
In its discussions and consideration of priorities and relevant subjects, the Steering Committee will invite the participation of representatives of the secretariat of national regulatory authorities of regional reference (NRAr), representatives of the Region's regulatory initiatives/frameworks, and associations of producers of health technologies, as well as FIFARMA and ALIFAR in their role as founding members.
PANDRH´s Steering Committee Members
Below are presented those who will represent the PANDRH Steering Committee until the 10th PANDRH Conference. Their roles and attributes are described in the PANDRH statutes:
SUBREGION
North America
Central America, Cuba, and Dominican Republic
Caribbean
Andean Region
Southern Cone
MAIN COUNTRY
United States
El Salvador
Suriname
Ecuador
Uruguay
ALTERNATIVE COUNTRY
Mexico
Costa Rica
Bahamas
Chile
Paraguay
SUBREGION
CRS
NRAs
ALIFAR*
FIFARMA*
MAIN COUNTRY
CARPHA
ANMAT
Rubén Abete
Rafael Diaz-Granados
ALTERNATIVE COUNTRY
N/A
N/A
Miguel Maito
María Fernanda Hurtado
*Founding Members
The Pan American Health Organization (Regional Office of the World Health Organization for the Americas PAHO/WHO) serves as the Secretariat of all the Network's components (Conference, Steering Committee, and technical structures). The Secretariat provides technical and administrative support to PANDRH.
Standards and Procedures
Consult the Statutes of the Pan American Network for Drug Regulatory Harmonization
PANDRH Technical Work
The PANDRH's technical work supports the implementation of the guidelines and decisions of the Pan American Conferences and Steering Committee through the development of projects in the areas identified as priorities by the Network.
During the VII Conference in Ottawa (Canada) in September 2013, the countries approved a PANDRH Strategic Development Plan for the period 2014-2020, where the need for the development of a systematic mechanism for priority work areas based on periodic analysis of the context and the needs of National Regulatory Authorities (NRAs) of each country is expressed.
The procedure should be applied by the Steering Committee of PANDRH for defining the biannual strategic thematic areas, where projects that include activities at achieving the aimed outcome of each of the subject areas defined as priority/strategic will be implemented. These areas should express regulatory functions or cross-cutting themes.
The strategic areas will be defined using the approved prioritization methodology and will be coordinated preferably by a national regulatory authority already designated as NRAs of regional reference (NRAr) based on Resolution CD 50.R9. Each of these areas will be made up of projects that will be proposed by any of the Members of the Network whose approval and implementation will be under the responsibility of the components of the strategic areas.
Participants in each of the strategic areas prioritized include other RNAs of the Region as long as they are interested in the subject, and representatives of manufacturer associations, civil society, and academia, as appropriate, according to the defined area. Each of the projects approved within the priority areas will be coordinated by one of the RNAs participating. An average of nine (9) participating Members by subject area (including RNA and other representatives) and the presentation of projects preceded by terms of reference presented to the Steering Committee which will consist of justification, activities, deadlines, resources, and expected results.
On this basis, the Steering Committee is going to monitor the development of these projects through regular reports by the leaders of the priority areas during face meetings of the PANDRH Steering Committee.
- Initiative of the National Regulatory Authorities for the joint evaluation of periodic safety update reports (PSURs), risk management plans (RMPs), and periodic benefit-risk evaluation reports (PBRERs). Proponent: Institute of Public Health of Chile, National Health Surveillance Agency (ANVISA-Brazil), and Health Canada
- Assessing CPP requirements for drug registration processes in the Region of the Americas towards more timely access to medicines and more convergent regulatory approaches. Initiator: Latin America Federation of the Pharmaceutical Industry -FIFARMA
- Foro permanente de regulación de biológicos de las Américas. Poponentes: Administración Nacional de Medicamentos, Alimentos y Tecnología Médica (ANMAT -Argentina) y Health Canada (conforme al consenso alcanzado en el Taller de regulación de biológicos en las Américas- Buenos Aires, octubre 2016) [available only in Spanish]/ Respuesta a comentarios efecutados sobre el Proyecto Foro permanente de regualación de biológicos de las Américas [available only in Spanish]
For information about former PANDRH working groups please visit the PANDRH Technical Working Groups Webpage.
They are groups of experts in areas that have been identified as priorities for drug regulatory harmonization. Members are selected by the Steering Committee and confirmed by the regulatory authorities of the respective countries. Whenever possible, WG should have at least one representative for each of the five sub-regional blocs of the Americas.
Academics and other experts can be members of the WG. Its main objectives include the implementation of diagnostic studies in order to identify the differences among the countries regarding the implementation of international standards and to define the necessary strategies for technical cooperation; analyze international guidelines and prepare harmonized proposals in their areas to be considered for the conference for its implementation in the region. The WG system was switched to a project-based approach on prioritized areas in 2015 [Terms of Reference. Procedure for the Prioritization of Areas and Selection of Projects PANDRH]
- PANDRH's rules and regulations, and a specific chapter on Working Groups operation. 17 November 2008 [updated]
- Member list of the Technical Working Groups (2010)
Member States promote harmonization and regulatory convergence through participation in PANDRH and the international harmonization mechanisms recommended by the Pan American Health Organization (PAHO) and World Health Organization (WHO) as sources of regulatory standards and good practices.”
CSP30.R12 - Policy to Strengthen National Regulatory Systems for Medicines and other Health Technologies
Main PANDRH' documents and PAHO/WHO Resolutions
Since PANDRH was established, it has produced documents pursuant to PAHO Resolutions and based on the needs of the countries of the Americas, supporting regional technical cooperation initiatives to strengthen national regulatory capacities
- Statutes of the Pan American Network for Drug Regulatory Harmonization. 2015
- Terms of Reference. Procedure for the Prioritization of Areas and Selection of Projects PANDRH. 2015
- PANDRH Strategic Development Plan 2014 - 2020 [Only in Spanish]
- 42nd Directing Council. Washington, D.C., 25-29 September 2000. Provisional Agenda Item 4.9 CD42/13, Rev. 1 (Eng.) 13 September 2000. Pharmaceutical Regulatory Harmonization in The Americas
- 42nd Directing Council. Washington, D.C., 25-29 September 2000. Resolution CD42.R11. Drug Regulatory Harmonization
- 55th Directing Council Access and Rational Use of Strategic and High-cost Medicines and Other Health Technologies (Resolution CD55.R12])
- CD55/10, Rev.1, - Access and Rational Use of Strategic and High-cost Medicines and Other Health Technologies
- 58th Directing Council. The virtual meeting, 28-29 September 2020). CD58/INF/14 - Progress Reports on Technical Matters: Strengthening National Regulatory Authorities for Medicines and Biologicals: Progress Report
- 59th Directing Council. The virtual meeting, Virtual Session, 20-24 September 2021). CD59.R3 Increasing Production Capacity for Essential Medicines and Health Technologies
- 59th Directing Council. The virtual meeting, Virtual Session, 20-24 September 2021). CD59/8 Increasing Production Capacity for Essential Medicines and Health Technologies
Steering Committee Members and Minutes
Conference and publications
The Conference of the Pan American Network on Drug Regulatory Harmonization (CPANDRH) is held every two or three years. The CPANDRH mission is "to promote drug regulatory harmonization for all aspects of quality, safety, and efficacy of pharmaceutical products as a contribution to the quality of life and health care of the citizens of the Member Countries of the Americas."
- XI Conference: Mexico City, Mexico (21- 23 October 2024)
- X Conference: Extraordinary Virtual Session (6, 8 and, 10 December 2021)
- IX Conference: San Salvador, El Salvador (24 - 26 October 2018)
- VIII Conference: Mexico City, Mexico (19 - 21 October 2016)
- VII Conference: Ottawa, Canada,(5 - 7 September 2013)
- VI Conference: Brasilia, Brazil (6 - 8 July 2011)
- V Conference: Buenos Aires, Argentina (17 - 19 November 2008)
- IV Conference: Dominican Republic (2 - 4 March 2005)
- III Conference: Washington, D.C. (24 - 26 April 2002)
- II Conference. Washington, D.C. (2 - 5 November 1999)
- I Conference: Washington, D.C. (18 - 20 November 1997)
They are groups of experts in areas that have been identified as priorities for the drug regulatory harmonization. Members are selected by the Steering Committee and confirmed by the regulatory authorities of the respective countries. Whenever possible, WG should have at least one representative for each of the five sub-regional blocs of the Americas.
Academics and other experts can be members of the WG. Its main objectives include the implementation of diagnostic studies to identify the differences among the countries regarding the implementation of internationals standards and to define the necessary strategies for technical cooperation; analyze international guidelines and prepare harmonized proposal in their areas to be considered for the conference for its implementation in the region.
During its existence, the TWG have contributed to the dissemination of technical knowledge consistent with the pharmaceutical regulatory harmonization in the Region of the Americas. These works, in major publications, are available in English, Spanish, and Portuguese. Some publications are material for historical reference because they have been updated or are in the process of being revised.
-
Regulatory reliance principles: concept note and recommendations. Ninth Conference of the Pan American Network for Drug Regulatory Harmonization (PANDRH) (San Salvador, 24 to 26 October, 2018). PANDRH 2019 (PAHO/HSS/19-003)
-
Regulation of Advanced Therapy Medicinal Products: Concept Note and Recommendations. Ninth Conference of the Pan American Network for Drug Regulatory Harmonization (PANDRH). (San Salvador, October 2018)
-
Regulatory System Models for Small States/Markets with Limited Resources. Concept Note and Recommendations. Ninth Conference of the Pan American Network for Drug Regulatory Harmonization (PANDRH). (San Salvador, 24 to 26 October, 2018)
-
PANDRH Network Technical Document Nº 1. Harmonized requirements for the licensing of vaccines in the Americas and Guidelines for preparation of application. (2010) 40 p
-
PANDRH Network Technical Document Nº 2. World Health Organization (WHO). Technical Report Series, No. 902, 2002. Inform 36, Annex 3. Good practices for National Pharmaceutical Control Laboratories
-
PANDRH Network Technical Document Nº 3. World Health Organization (WHO). Good practices self-evaluation guide for National Pharmaceutical Control Laboratories
-
PANDRH Network Technical Document Nº 4. World Health Organization (WHO). Study of the Current Conditions of the Official Medicine Control Laboratories (OMCL) in Latin America and the Caribbean
-
PANDRH Network Technical Document Nº 5. Good Pharmacovigilance Practices for the Americas (2011) 83p.
-
PANDRH Network Technical Document Nº 6. Document on Self-Evaluation of Good Laboratory Practices (GLP)
-
PANDRH Network Technical Document Nº 7. Recomendaciones para la Evaluación de Productos Bioterapéuticos Similares (PBS) (2011) 82 p. (Spanish and Portuguese only)
-
PANDRH Network Technical Document Nº 8. Framework for Implementation of Equivalence Requirements for Pharmaceutical Products (2011) 38 p.
-
PANDRH Network Technical Document Nº 9. Boas Práticas da OMS para Laboratórios de Controle de Qualidade de Produtos Farmacêuticos (2011) 58. (Portuguese only)
-
PANDRH Network Technical Document Nº 10. Requirements for Medicines Registration in the Americas (2013) 42 p.
-
PANDRH Network Technical Document Nº 12. Ethical Criteria for the Promotion, Advertisement, and Publicity of Medicines (2013) 16 p.
-
PANDRH Network Technical Document Nº 14. Strategic Development Plan 2014-2020 of the Pan American Network for Drug Regulatory Harmonization