Influenza and SARS-CoV-2 integrated surveillance laboratory testing algorithm
|
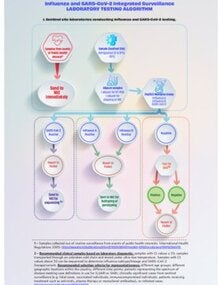
The integrated surveillance algorithm for influenza, SARS-CoV-2, and other respiratory viruses standardizes diagnostic, molecular, and genomic procedures within national surveillance systems. Its operational flow ranges from sample collection and transport at sentinel sites to detection by multiplex RT-PCR, subtyping or genotyping at National Influenza Centers (NICs), and genomic sequencing for phylogenetic analysis.
|
