BANVACO: A New Era in Animal Health Protection for the Americas
“Si vis pacem, para bellum”
Foot-and-mouth disease (FMD) does not affect humans, but it is one of the most contagious animal diseases, capable of infecting cattle, buffalo, pigs, sheep, and goats. It threatens global food supply and causes devastating economic and social impacts.
Despite decades without cases in the Americas, the threat persists. Globalization, high travel volumes, and outbreaks in other parts of the world keep the risk alive, whether through natural reintroduction or as a result of bioterrorism. International experience shows that the best defense is advance preparedness—and for FMD, that means being ready to apply emergency vaccination whenever necessary.
Emergency vaccination is globally recognized for reducing economic losses and for being more socially acceptable than mass culling. It also prevents the waste of animal protein and allows rapid restoration of official disease-free status. However, such a response is only possible with prior planning, public–private coordination, and guaranteed rapid access to suitable vaccines, including against viral strains not currently present in the region.
With this forward-looking vision, the Regional Foot-and-Mouth Disease Antigen Bank – BANVACO was created—an unprecedented initiative in the Americas to ensure immediate availability of antigens and vaccines both for viruses eradicated from the continent and for exotic strains from outside the region.
BANVACO’s founding governing board includes Ecuador’s Agency for Phytosanitary and Zoosanitary Regulation and Control (AGROCALIDAD), Paraguay’s National Animal Health and Quality Service (SENACSA), and Brazil’s Animal Health Department (DSA/MAPA). This continental network marks a milestone in animal health security and in strengthening livestock production across the Americas.
BANVACO is managed by the Pan American Center for Foot-and-Mouth Disease and Veterinary Public Health (PANAFTOSA/VPH) of the Pan American Health Organization (PAHO/WHO), ensuring neutrality, technical expertise, and logistical capacity for acquiring, storing, and distributing antigens and vaccines. This strategic tool will allow fast, coordinated responses to any reintroduction of FMD—preserving markets, protecting herds, and safeguarding the sustainability of livestock production.
Today, with near of 98% of cattle in the Americas free from the disease, BANVACO represents more than just a technical achievement—it is a collective pact to protect animal health and ensure the continent’s economic and food security.
All FMD-free countries—whether they vaccinate or not—face the same risk: the reappearance of an outbreak. While historical data suggests this probability is very low in the Americas, the threat cannot be dismissed. The disease has not been completely eradicated from the region and remains endemic in many countries in Africa, Asia, and the Middle East. The possibility of a bioterrorist attack also cannot be ignored, as several countries worldwide consider it a plausible scenario.
What is the objective of BANVACO?
The Bank’s goal is to ensure the effective availability of antigens and vaccines to contain outbreaks of foot-and-mouth disease in populations currently free from infection in the Americas, as per the World Organisation for Animal Health (WOAH) Terrestrial Animal Health Code. It also aims to maintain antigen reserves for all viral serotypes of foot-and-mouth disease that currently pose a risk to BANVACO members, so they can be used in emergency vaccination in the control of a potential outbreak.
Who can belong to BANVACO?
All PAHO member countries can join, not only COSALFA members countries. FMD-free countries with vaccination can also be members of the Regional Foot-and-Mouth Disease Antigen Bank – BANVACO.
QUESTIONS AND ANSWERS
In 2012, during the XII Meeting of the Hemispheric Committee for the Eradication of Foot and Mouth Disease (COHEFA), the countries of the Americas requested PANAFTOSA to conduct a technical study with the participation of experts from the countries of the region for the future creation of a regional antigen/vaccine bank.
The final proposal for the establishment of the Regional Bank of Foot-and- Mouth Disease Antigens - BANVACO - was completed in December 2018 and distributed to all member countries in 2019. More information at https://www.paho.org/es/panaftosa/banvaco.
Emergency vaccination is a universally accepted measure for controlling foot-and-mouth disease outbreaks because it can reduce economic losses.
A foot-and-mouth disease–free country without vaccination has six available control strategies in the event of an outbreak, depending on the decisions made regarding the two mentioned health measures. In contrast, a disease-free country with vaccination has three control strategies available.
However, if a country decides not to use emergency vaccination to control an outbreak, the available control strategies are reduced to two in the case of free countries without vaccination, and to one for disease- free countries with vaccination. This highlights the critical importance of having the option to implement emergency vaccination in the event of a a foot-and-mouth disease outbreak in a free country. Therefore, having the option of using emergency vaccination requires a series of actions and coordinated efforts well in advance of outbreak occurs.
A vaccine bank is therefore essential to support countries in controlling an outbreak more effectively, reducing its impact and the severe economic and social consequences of the disease.
Foot and mouth disease can be caused by seven distinct viral serotypes, which do not confer cross-immunity, in addition, multiple subtypes have been identified within some of these serotypes. Therefore, vaccines used in an emergency must be serotype-specific and in certain cases subtype-specific.
Therefore, when faced with an outbreak of foot-and-mouth disease, the serotype and subtype involved must be identified so that, in the event that emergency vaccination is used, the vaccine that produces protection in animals can be verified. The vaccine must then be produced, distributed and administered to animals to help contain the outbreak. In particular, once the vaccine strain that produces immunity has been identified, a laboratory that has the vaccine strain must be used to produce the vaccine in a short period of time in the quantity that the country requires to control the outbreak. This entire process takes time.
Free countries have solved this problem by creating reserve banks of antigens and vaccines.
A vaccine antigen bank is a strategic reserve of frozen viral antigen concentrate of specific viral serotypes that a laboratory can rapidly formulate into a vaccine in the event of a disease outbreak.
The terms "vaccine bank" and "antigen bank" are often used interchangeably. However, the antigen for a foot-and-mouth disease vaccine is the inactivated virus that is kept frozen and formulated into a vaccine only when needed.
Research has shown that viral antigens can be inactivated, concentrated and stored at low temperatures for extended periods with minimal or no loss of immunogenicity created the basis for the establishment of Antigen Banks, which can be rapidly reconstituted and formulated into potent FMD vaccines within 4–14 days.
Ready-to-use FMD vaccines have a short shelf life, whereas frozen antigen concentrate can be stored for many years. It is neither efficient nor cost-effective to preventively formulate and store vaccines that may not be used, especially since it is impossible to predict which viral strains may emerge.
Antigen banks provide flexibility to formulate targeted vaccines for any specific FMD strain in any specific outbreak.
The first national antigen bank against foot and mouth disease was established by Denmark in 1976.
In 1982, the North American Vaccine Bank (NAVB) was established with the participation of the United States, Mexico and Canada.
In 1985 the International Vaccine Bank (IVB) was established in Pirbright, Surrey, to support the United Kingdom, Australia, New Zealand, Finland, Ireland, Norway and Sweden during foot- and- mouth disease emergencies. The IVB operates as a non-commercial, intergovernmental entity combining antigen conservation with the capacity to formulate and test vaccines. Member countries pay an annual subscription to cover maintenance costs, which includes the acquisition of new antigens and the replacement of those withdrawn.
The European Union Vaccine Bank (EUVB) was established in 1991 to serve its member states. It holds the equivalent of 500 to one million doses of various vaccine strains stored, at two national institutes located in two different countries.
These three banks are known to maintain reserves of antigens for all serotypes; however, for reasons of national security, the details of the stored reserves were not published. In addition to these international banks, according to data published in Europe, 13 countries maintain additional antigen reserves.
In South America, where all countries are free of foot-and-mouth disease with or without vaccination, except for Venezuela, there is only one antigen bank, located in Argentina and established 1999. This bank holds reserves of the viral serotypes that have been endemic in the region, as well as some extra-regional serotypes. However, this private antigen bank was created only to control an outbreak in Argentine territory.
In summary, the foot-and-mouth disease-free countries on the continent, with the exception of Argentina, the United States and Canada, do not have reserves of foot-and-mouth disease antigens that would enable them to formulate vaccines for emergency vaccination in response to any serotype of the foot-and-mouth disease virus.
BANVACO is not a new physical facility or a legal entity. Its management is based at PANAFTOSA/SPV-PAHO/WHO headquarters, while member countries’ antigen and vaccine stocks are stored at regional vaccine suppliers’ facilities under specific contracts
The Pan American Health Organization (PAHO), through PANAFTOSA/SPV, was chosen for its supranational status, neutrality, extensive FMD expertise, and proven ability to procure, maintain, and distribute antigens and vaccines in both human and animal health contexts.
For over 40 years, PAHO’s Revolving Fund for Vaccine Access has provided safe, high-quality vaccines at affordable prices to member states and territories. By consolidating demand, leveraging economies of scale, negotiating transparently with suppliers, and implementing innovative procurement strategies, the Fund has reduced costs and improved sustainability for national immunization programs.
Through this system, 41 countries in the Americas access safe, high-quality human vaccines at prices up to 75% lower than if purchased individually.
All PAHO countries members can join BANVACO, sharing the bank’s fixed administrative costs. The more countries join, the lower the fixed cost for each. The BANVACO Governing Board, composed of a delegate from each member country, determines the percentage of the annual budget that corresponds to each member country. Initially, the fixed costs were calculated at USD 25,000 per member country for the first year, with the possibility of reducing this amount as additional countries join and upon approval of the annual work plans.
Variable costs depend on the stock of antigens each country decides to maintain, based on its own needs for emergency vaccine production.
Pooling antigen acquisition and storage at an international level allows economies of scale, operational synergies, and better global and regional coordination for FMD emergency preparedness.
Absolutly not.
FMD-free countries with vaccination should also join BANVACO, since the goal is to maintain antigen reserves for all viral serotypes that could threaten the continent, for use in emergency vaccination during an outbreak.
In fact, the first two signatories—Paraguay (2021) and Ecuador (2023)—are both countries that systematically vaccinate their animals.
Yes. There is no restriction. In fact, many FMD-free countries in North America and Europe have their own reserves in addition to regional bank membership.
Membership in a regional bank is strategically important because FMD prevention must be addressed from a regional perspective.
Not necessarily
Emergency vaccination can be implemented to protect healthy animals from spreading the virus outside the containment area, including in international border regions, in the event of foot-and-mouth disease outbreaks in neighbouring countries.
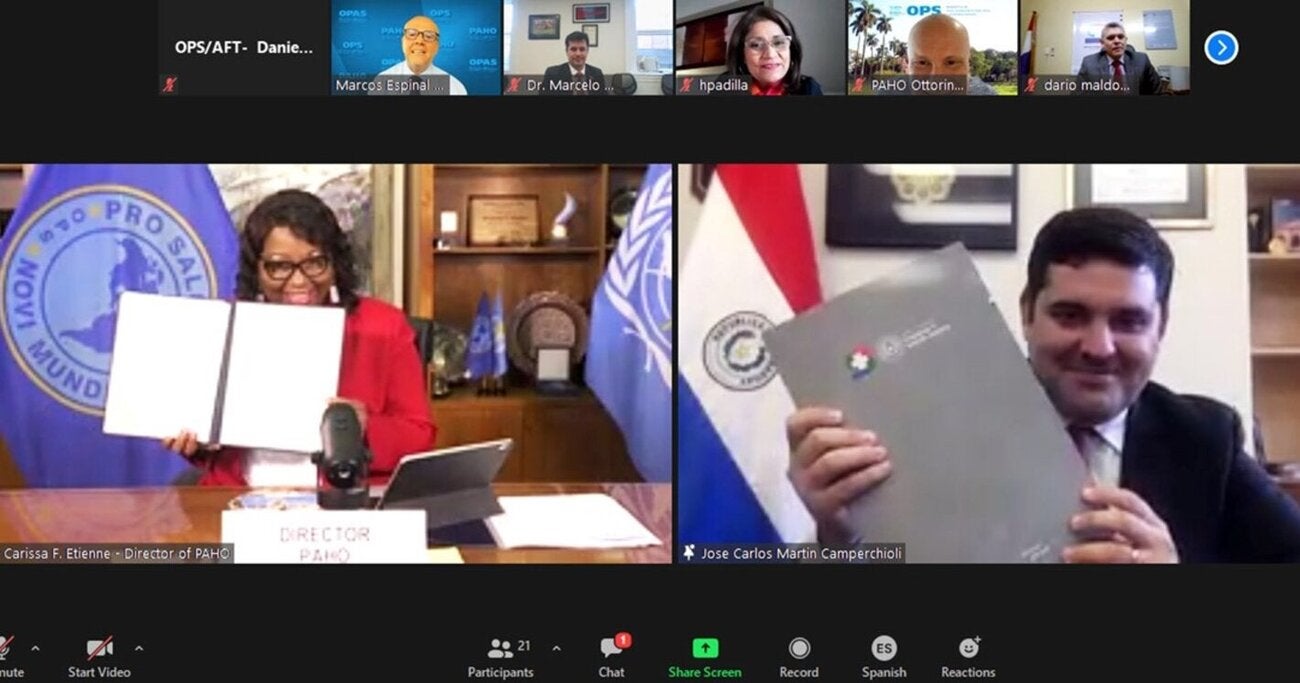
Paraguay Becomes the First South American Country to Sign the Constitutive Agreement of BANVACO
Paraguay is the first country in South America to sign the Constitutive Agreement of the Regional Vaccine Bank against Foot-and-Mouth Disease (BANVACO), which requires the participation of at least three countries to begin its operations as a regional initiative.
Dr. Carissa Etienne, Director of PAHO, emphasized the significance of this agreement.
“The current risk of foot-and-mouth disease for free countries lies in the potential introduction of the virus from one of the eight affected regions in the world, where various viral types and subtypes continue to circulate,” she stated. “That is why the work of PANAFTOSA/SPV-PAHO/WHO, which has been fighting this disease for over 70 years, is so crucial. We will make BANVACO a success story.”
The President of SENACSA-Paraguay, Dr. José Carlos Martin, highlighted the strategic importance of this step:
“Today we are taking a truly important and strategic step in the process of transitioning from a ‘free with vaccination’ status to a ‘free without vaccination’ status, following the recommendations of the PHEFA Plan. In this context, I want to acknowledge Paraguay’s leadership in being the first to join this initiative.”
The Pan American Foot-and-Mouth Disease and Veterinary Public Health Center of the Pan American Health Organization (PANAFTOSA/SPV-PAHO/WHO) is a specialized center within PAHO that, since its establishment in 1951, has cooperated with and provided assistance to countries across the Americas on measures for the control and eradication of foot-and-mouth disease in the region, as well as on the implementation and maintenance of disease prevention programs aimed at eliminating the risk of its reintroduction.
In December 2018, following several technical discussions and legal analyses among the member countries of COSALFA, the final draft of the constitutive agreement for adhesion was sent to all countries. The management of BANVACO was requested by the member countries from PAHO through PANAFTOSA/SPV-PAHO/WHO to ensure its status as a supranational and neutral entity and to leverage its extensive experience in the procurement and maintenance of antigens and vaccines, processes already established by this organization.
Countries in the Americas that meet the conditions set forth in the constitutive agreement to be signed may become members of BANVACO.
Members
Delegate
Marcelo de Andrade Motta
Director, Animal Health Department – MAPA
Ministry of Agriculture, Livestock, and Supply (MAPA)
Commission Members
Bruno de Oliveira Cotta
General Coordinator of Animal Transit and Quarantine
Delegate
Christian Antonio Zambrano Pesantez
General Coordinator of Animal Health – AGROCALIDAD
Agency for Phytosanitary and Zoosanitary Regulation and Control (AGROCALIDAD)
Commission Members
Luz Jacqueline Aguilar Narváez
Animal Disease Managment and Control Specialist
Delegate
Primo Ricardo Feltes
Director General of Technical Services
National Service for Animal Quality and Health (SENACSA)
Commission Members
Elizabeth Oviedo
Director of Laboratories
Delegate
Edgardo Vitale
Director of the Department of Health Programs (Epidemiology Unit)
Ministry of Agriculture, Livestock, and Fisheries
Commission Members
Glória Arnaud
Deputy Director of the Animal Health
Delegate
Carlos Edson Peñaranda Bersatti
Private sector of Bolivia
Inter-American Group for the Eradication of Foot-and-Mouth Disease (GIEFA)
Commission Members
Rodrigo Miguel Garcia Muñoz
Private sector of Paraguay
Delegate
Ottorino Cosivi
PANAFTOSA/VPH-PAHO/WHO Director
Pan American Center for Foot-and-Mouth Disease and Veterinary Public Health
Integrantes de la Comisión
Foot-and-Mouth Disease Advisor, PANAFTOSA/VPH-PAHO/WHO