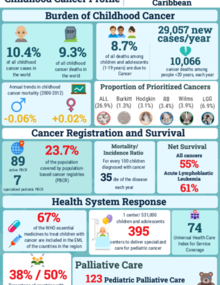
Cancer is a leading cause of death in the Americas. In 2022, cancer accounted for 1.4 million deaths, 45.1% of which occurred in people of 69 years-old or younger.
The number of cancer cases in the Americas in 2020 is estimated in 4.2 million cases, and it is projected to increase to 6 million in 2045, representing an increase in 59.3%.
About a third of all cancer cases could be prevented by avoiding key risk factors. These include tobacco use, harmful use of alcohol, unhealthy diet and physical inactivity. Vaccination and screening programmes are effective interventions to reduce the burden of specific types of cancer. Many cancers have a high chance of cure if detected early and treated adequately.
In the Region of the Americas:
- The most frequently diagnosed types of cancer in men are: prostate (8.6%), lung (11.7%), colorectal (10.2%) and bladder (5.9%).
- The most frequently diagnosed types of cancer in women are: breast (30.7%), lung (10.3%), colorectal (9.6%) and uterus (6.4%).
- The types of cancer with the highest mortality rates in men are: lung (20.6%), prostate (14.5%), colorectal (10.6%), pancreas (7.0%) and liver (6.6%).
- The cancers that cause the highest number of deaths in women are: lung (18.4%), breast (17.5%), colorectal (10.6%) and pancreas (7.2%).
- Almost 500,000 new cases of breast cancer and more than 100,000 deaths from breast cancer were registered in the region.
In Latin America and the Caribbean:
- More than 56,000 women were diagnosed with cervical cancer in Latin America and the Caribbean and more than 28,000 lost their lives.
All people, both collectively and individually, can contribute to reducing the cancer burden.
Individually, people can:
- Talk to their doctor about their risk of cancer and how to reduce it
- Participate in cancer screening and early detection programs
- Adopt healthy lifestyles to reduce cancer risks
- Advocate for better access to cancer screening and treatment services
- If they or a loved one has had cancer, share their experiences with others
- Support people living with cancer
- Understand that early detection and early treatment save lives.
Collectively, we can:
- Prevent cancer
- Detect cancer early and improve treatment results
- Create health-promoting public policies to reduce cancer risk factors
- Ensure palliative care policies and access to pain medications
- Improve access to cancer care and cancer treatments
- Demonstrate the need to invest in cancer detection, treatment and research
- Better understand the causes and risk factors of cancer and how to prevent it
- Train health professionals to join the fight against cancer
- Educate and inform the public about cancer.
PAHO is working with countries in the Americas to reduce premature deaths from non-communicable diseases (NCDs), including cancer, by 25% by the year 2025. Through PAHO's NCD plan of action, launched in 2013, PAHO promotes the reduction of tobacco use and harmful use of alcohol, promotes healthy diet and increased physical activity, supports the introduction of HPV vaccines and HPV testing to prevent cervical cancer, and promotes improvements in quality and access to early diagnosis of breast cancer. PAHO also supports improvements in radiotherapy services and access to affordable essential chemotherapy drugs; and promotes the expansion of palliative care policies, programs and opioids for pain relief and symptom management.
Strategies proposed by PAHO/WHO to reduce the risk of cancer and other noncommunicable diseases include:
- Increasing taxes, restricting access, and warning about the dangers of tobacco and harmful use of alcohol
- Promoting public awareness about healthy diet, physical activity and healthy weight
- Immunizing infants against hepatitis B to prevent liver cancer, and immunizing girls against human papillomavirus to prevent cervical cancer
- Organizing screening programs for cervical cancer and breast cancer to detect them at early stages, when they are more amenable to treatment