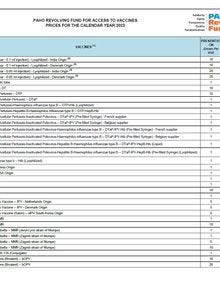
For more than 40 years, PAHO’s Revolving Fund for Access to Vaccines has provided Member States and Territories throughout the region with access to safe, quality vaccines at affordable prices. It is part of PAHO's larger technical cooperation package that supports countries' efforts to achieve sustainable reductions in the morbidity and mortality of vaccine-preventable diseases through control and elimination strategies.
By consolidating forecasted demand requirements and leveraging economies of scale, promoting transparent negotiations with suppliers, and implementing innovative acquisition strategies, the Revolving Fund greatly improves its purchasing power, lowering vaccine prices and contributing to the sustainability of National Immunization Programs.