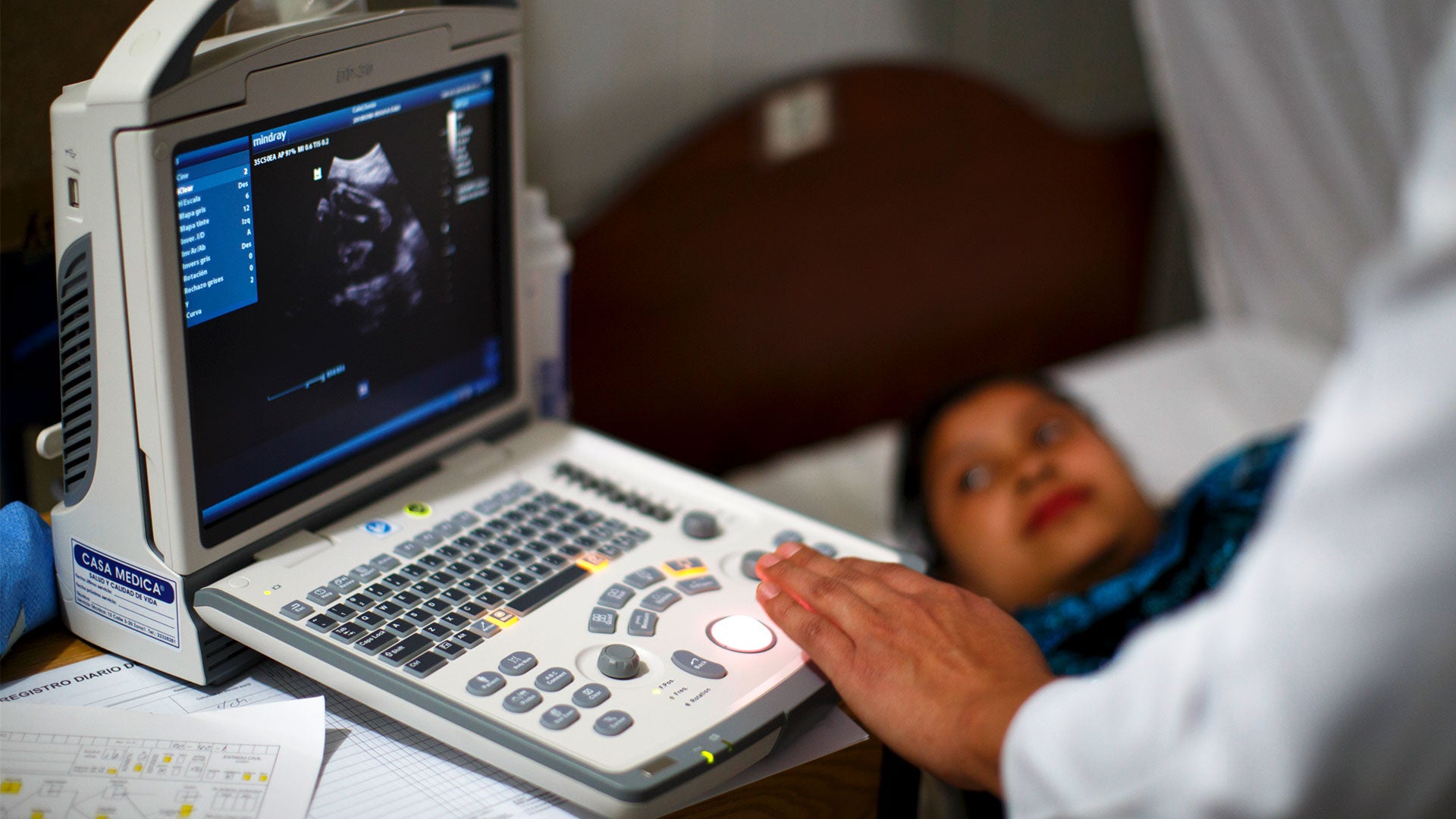
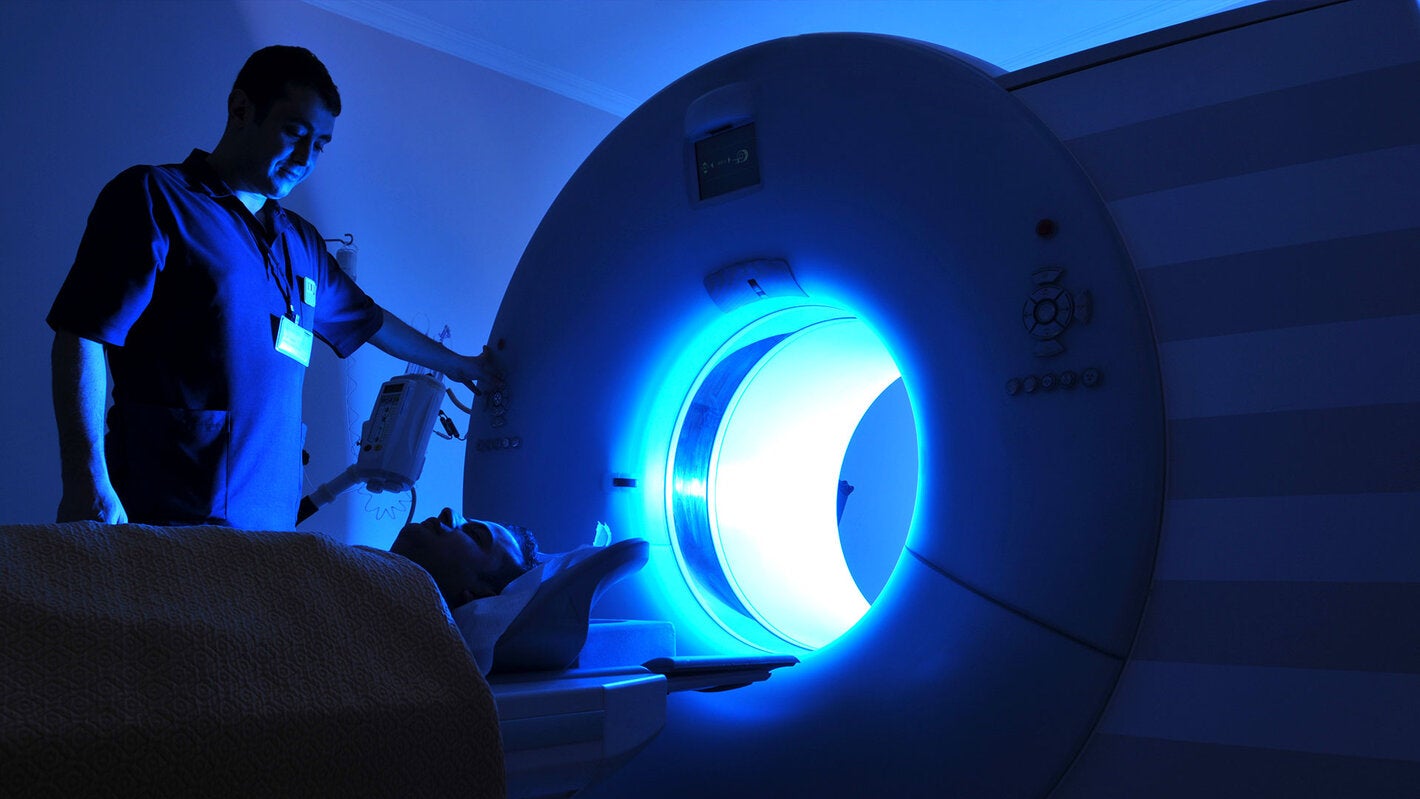
An assessment report gives information about how the technology can be adapted to current available technologies and the added value of the reviewed technology to the current technologies. This final assessment contains evidence-based conclusions with the outcomes of the use of the technology supporting the decision-making process of health policymakers. Health technologies include pharmaceuticals, equipment and machines, as well as procedures and physical techniques for health prevention and promotion.
In September 2012, the Member States were pioneers when they adopted the first resolution on health technology assessment (HTA) and the incorporation of health technologies into health systems. A resolution was adopted an innovative policy paper that proposes linking HTA with the decision-making processes involved in incorporating these technologies into health systems.
The Region was mapped to determine the status of HTA. The responses from the countries indicate that the Region has 76 institutions that carry out some type of HTA-related activity: 49% of them are governmental and 34% are academic institutions.
Health Technology Assessment (HTA) is the systematic evaluation of properties, effects, and/or impacts of health care technology. It should include medical, social, ethical, and economic dimensions, and its main purpose is to inform decision-making in the health area. These assessments look at benefits and efficacy, clinical and technical safety, and cost-effectiveness. Informed decision-making comprises issues surrounding coverage and reimbursement, pricing decisions, clinical guidelines and protocols, and lastly, medical device regulation. The main purpose of HTA is to inform a policy decision making in health care, and thus improve the uptake of cost-effective new technologies and prevent the uptake of technologies that are of doubtful value for the health system.
HTA is used to define which benefits to include while carrying out evidence-based assessments. New technologies are usually costlier than older ones and contribute to rising health expenditures. In this context, the HTA process ensures that new technology is not added until it is proven to be effective. Meanwhile, older technology is not removed from the health package until it is shown to be ineffective or not cost-effective. HTA is also concerned with quality, and the role of new technologies to improve health outcomes. PAHO supports HTA activities that emphasize health outcomes that can be measured against a benchmark.
Given the growing interest for health technologies, PAHO has launched several initiatives in HTA with member countries to promote and strengthen health technology assessment in the Americas. PAHO's role is important for the development and implementation of HTA in the Americas, and to support the promotion of evidence-based decision-making processes, which contribute to the incorporation of cost-effective technologies.
In 2012 member states adopted the resolution "Health Technology Assessment and incorporation into Health Systems" (CSP28.R9). The resolution proposes linking HTA with the decision-making processes involved in incorporating these technologies into health systems. Since the approval of CSP28.R9, there have been clear advances in the institutionalization of HTA in the Region, both at regional and national levels. Despite the major progress, the implementation of HTA remains at a low level in some countries. PAHO encourages the establishment of an institutional framework for HTA-based decision-making. This framework would establish linkages between HTA and decision-makers, encouraging institutional responsibility and creating linkages between the use of technologies and evaluative data to feed into the decision-making process.
To support the development of HTA in member countries, the Medicines and Health Technologies Unit is involved in many activities, such as:
- Human resources development: establishing a regional strategy to assess different regional needs. Promotion of regional meetings, workshops, and training through online courses, and webinars.
- Dissemination of information: identifying existing opportunities and disseminating findings amongst stakeholders and decision-makers, through the Regional Platform on Access and Innovation for Health Technologies (PRAIS).
- Rational use of health technologies: developing and implementing clinical guidelines to evaluate the use of health technologies in health services.
- Promotion of network collaboration: promotion of regional cooperation among member countries, strengthening the HTA Regional Network through the Regional Network of Health Technology Assessments for the Americas (RedETSA).