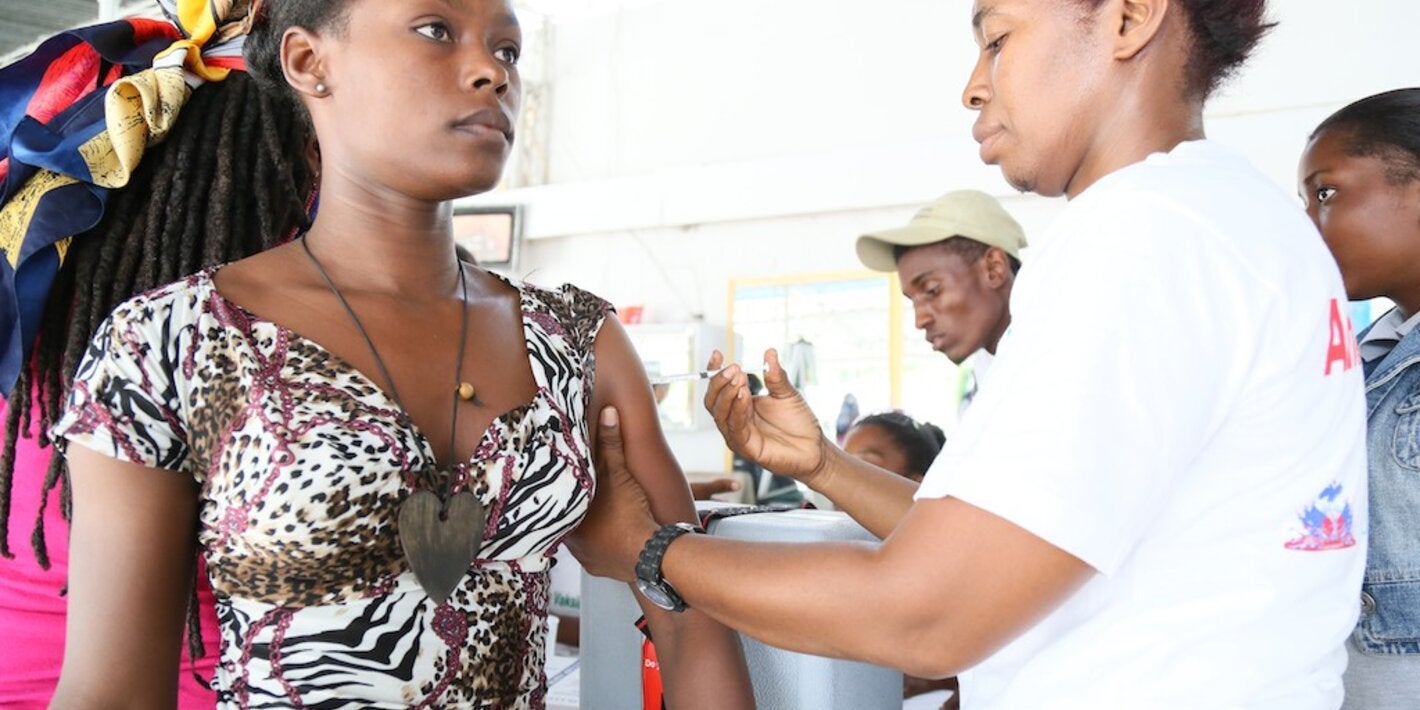
The administration of Tetanus Toxoid Containing Vaccines (TTCVs) is the most cost-effective measure for preventing maternal and neonatal tetanus (MNT) and injury-associated tetanus. These vaccines are safe and affordable, and should be administered according to the WHO recommended schedule of three primary infant series followed by three booster doses at 12 – 23 months, 4 – 7 years, and 9 – 15 years.
Different national schedules are in use for the 3-dose primary paediatric series, including vaccination at the following ages: 6, 10, and 14 weeks; 2, 3, and 4 months; 3, 4, and 5 months; and 2, 4, and 6 months. The tetanus toxoid (TT) is available as a single-antigen vaccine and in combination vaccines to protect against other vaccine preventable diseases, including diphtheria, pertussis, poliomyelitis, hepatitis B, and illness caused by Haemophilus influenzae type b (Hib).
The pentavalent vaccine, which provides protection against diphtheria, tetanus, pertussis, hepatitis B, and Hib (DTP- HepB - Hib), is the most commonly used childhood vaccine worldwide, but other pentavalent (DTaP-IPV/Hib) and hexavalent (DTaP/DTwP- HepB-Hib-IPV) combinations are also available. For booster dosing, a tetanus-diphtheria combination with a lower concentration of diphtheria antigen (Td) is available. As of December 2023, nearly all countries have replaced TT with Td in their vaccination schedules based on WHO recommendations. The TT is also used as a carrier protein in some conjugate vaccines, including Hib, meningococcal (A, C, ACYW, and combinations C-Hib, CY-Hib), pneumococcal (PCV), and typhoid (TCV) conjugate vaccines.