The XI Conference of the Pan American Network for Drug Regulatory Harmonization (PARF Network) recommended strengthening the technical capacities of National Regulatory Authorities (NRAs). To this end, the Regional Forum of Regulators was formed to oversee clinical trials.
Objectives:
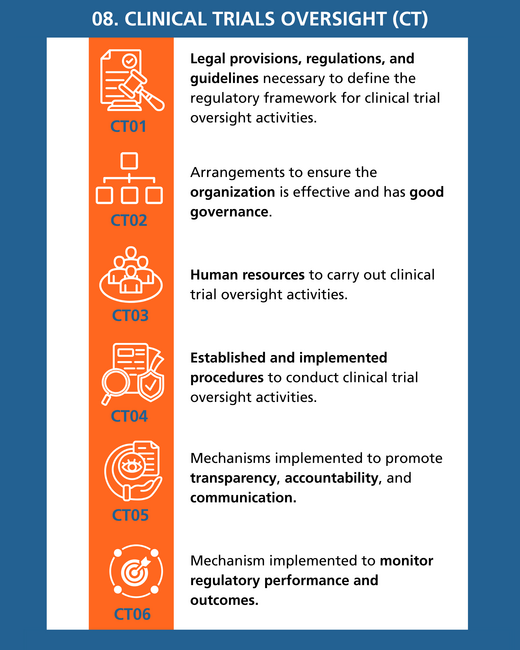
- Exchange experiences regarding the indicators of the WHO Global Benchmarking Tool for evaluating national regulatory systems for medical products, specifically related to the regulatory function of clinical trial oversight.
- Promote knowledge sharing among technical experts from NRAs.
- Encourage training, deepening, and development of high-impact topics to facilitate the effective implementation of this regulatory function.
:: Affiliated members in 2025
All National Regulatory Agencies are formally invited to join this Forum, conceived as a space for technical exchange and peer-to-peer cooperation.
To date, the following National Regulatory Agencies have joined the Forum as technical members in clinical trial oversight: