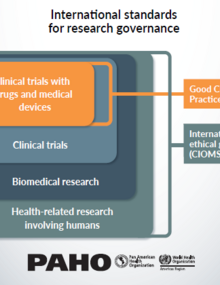
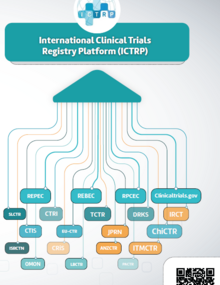
In some cases, national regulatory authorities (NRAs) do not exist or have a limited scope of work. In others, their requirements and procedures for the authorization and control of clinical trials do not adhere to international standards and become practical obstacles. These hurdles are even more complex when a trial must comply with regulations of different NRAs at different maturity levels, and there are no adequate channels of communication and coordination between the NRA and the investigators, RECs, or other authorities involved in conducting clinical trials.
Several research ethics committees (RECs) must review the same trial. Numerous reviews by different RECs within the same jurisdiction (i.e., country, state) are often necessary, which delays the processes to launch trials without necessarily strengthening them from an ethics perspective. Additional reviews are further needed from different jurisdictions where trials are being conducted.
Discussion questions:
- What is the best strategy to ensure rigorous yet efficient ethics review of clinical trials in the region, i.e., within specific jurisdictions and across Latin American and Caribbean countries?
- A “single IRB” policy has been implemented recently in the US. Can a similar policy be developed for the needs of Latin American and Caribbean countries, and implemented successfully, considering the lessons learned in the US and prior experience centralizing ethics review processes in Latin America?
- Are there other strategies that should be explored to avoid repetitive ethics review processes, e.g., adopting novel mechanisms for review that have been used in Argentina during COVID or establishing an extra-territorial ethics review committee, at least in certain jurisdictions like the Caribbean?