Hepatitis vaccines
Hepatitis B and hepatitis A are preventable with currently available safe and effective vaccines. A combined vaccine that provides protection against both hepatitis A and hepatitis B is also available.
Hepatitis B Vaccine
The hepatitis B vaccine offers a 95-100% protection against hepatitis B. Preventing hepatitis B virus (HBV) infection averts the development of complications including chronic disease and liver cancer. It was the first vaccine to prevent cancer.
The virus is transmitted from mother to child during birth and delivery and through contact with blood or other body fluids. The likelihood that infection becomes chronic depends on the age at which a person becomes infected (80–90% of infants infected during the first year of life and 30–50% of children infected before the age of 6 years). Vaccination with a birth dose of hepatitis B within the first 24 hours of life followed by infant vaccination series aims to prevent mother to child transmission and to create community-wide immunity to hepatitis B.
Mandates
In alignment with WHO recommendations, PAHO's Technical Advisory Group on Vaccine-Preventable Diseases (TAG) has recommended countries of the Region to vaccinate all newborns with a birth dose of hepatitis B vaccine administered preferably within 24 hours, followed by two or three doses during the first year of life. For routine infant vaccination, countries can use monovalent hepatitis B vaccines or combined vaccines containing hepatitis B
The vaccine is also recommended for people not previously vaccinated, who are in high-risk groups to acquire the infection. This includes:
- people who frequently require blood or blood products, dialysis patients and recipients of solid organ transplantations;
- people in prisons;
- people who inject drugs;
- household and sexual contacts of people with chronic HBV infection;
- people with multiple sexual partners;
- healthcare workers and others who may be exposed to blood and blood products through their work; and
- travelers who have not completed their HBV series, who should be offered the vaccine before leaving for endemic areas.
The vaccine has been approved for use during pregnancy and is highly recommended for pregnant women who have not been previously immunized.
Elimination of hepatitis B mother to child and early childhood transmission in the Americas
In 2016, based on progress made by countries and a modeling exercise, the TAG assessed that elimination of mother-to-child and early childhood horizontal transmission of hepatitis B (prevalence ≤0.1% in children aged 5 years) was feasible in the Americas by 2020 with high vaccination coverage of hepatitis B birth dose and infant hepatitis B vaccination (three doses).
The current low prevalence of chronic HBV infection in children under 5 years of age in the Americas (estimated at lower of 0.1% in 2019, the lowest in the world), can be attributed to the widespread use of hepatitis B vaccine in the Region:
- All 51 countries and territories in the Americas have introduced hepatitis B vaccine (or hepatitis B containing vaccine) in their routine immunization schedules, with 81% of regional coverage with three doses among children less than one year of age in 2019.
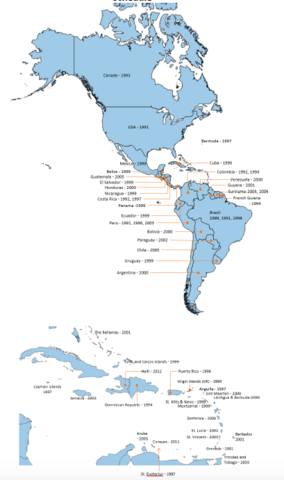
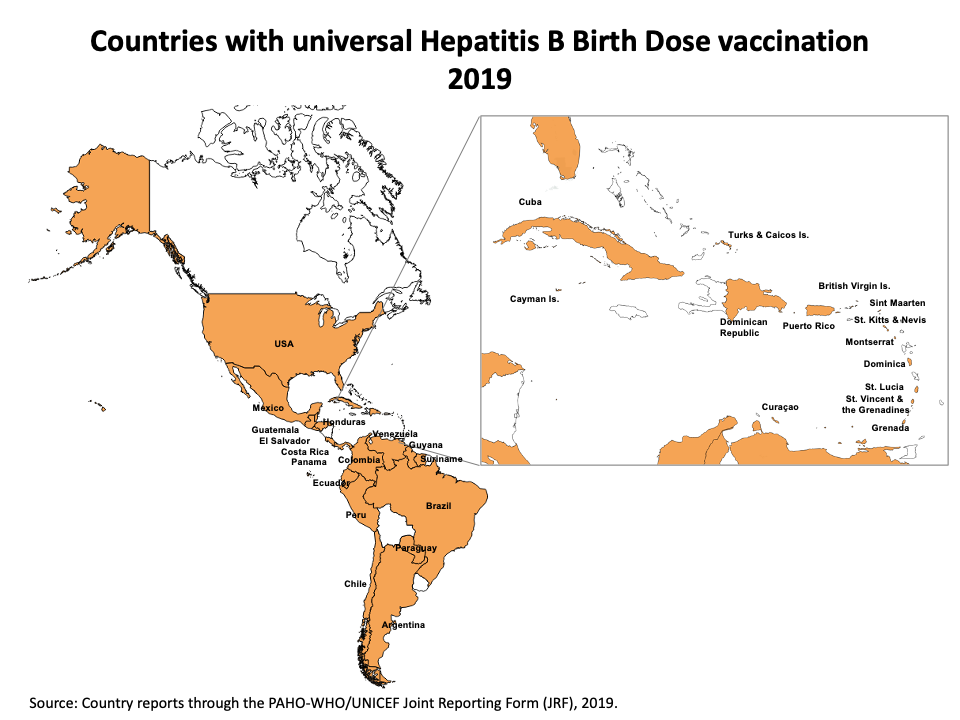
- In line with the targets of the Regional Immunization Action Plan (RIAP) 2016-20 important progress has also been made on the introduction of universal hepatitis B birth dose, from 18 countries in 2013 to 31 countries in 2019 with regional coverage of 72%.
PAHO's technical cooperation aims to:
- Provide guidance to national immunization programs for the introduction of universal hepatitis B birth dose and for maintenance of high infant vaccination coverage.
- Provide access to affordable monovalent hepatitis B and combined vaccines containing hepatitis B for countries in the Americas through the Revolving Fund.
- Monitor and reports progress towards hepatitis B regional elimination targets.
- In coordination with WHO, contribute to developing methodologies, guidance and a process for the validation of mother-to-child and early childhood horizontal hepatitis B elimination in the countries in the Americas.
Infant Hep B vaccination
Hepatitis B Vaccine Coverage
PAHO: Immunizations
PAHO: Hepatitis
WHO: Hepatitis Vaccine
Hepatitis A Vaccine
Nearly 100% of people develop protective levels of antibodies to the virus within 1 month after the injection of a single dose of vaccine. Several injectable inactivated hepatitis A vaccines are available. No vaccine is licensed for children younger than 1 year of age. Anyone who has not been vaccinated or previously infected can get infected with the hepatitis A virus. In areas where the virus is widespread (high endemicity), most hepatitis A infections occur during early childhood.
Mandates:
In alignment with WHO recommendations, PAHO’s TAG on Vaccine-Preventable Diseases encouraged countries to conduct their own epidemiological and cost-effectiveness studies to inform evidence-based decisions about the introduction of hepatitis A vaccination in their regular childhood immunization schedule.
Whether or not to include the vaccine in routine childhood immunizations depends on the local context. The proportion of susceptible people in the population and the level of exposure to the virus should be considered. Generally speaking, countries with intermediate endemicity will benefit the most from universal immunization of children. Countries with low endemicity may consider vaccinating high-risk adults:
- users of recreational drugs;
- travelers to countries where the virus is endemic;
- men who have sex with men; and
- people with chronic liver disease (because of their increased risk of serious complications if they acquire hepatitis A infection).
In countries with high endemicity, the use of vaccines is limited as most adults are naturally immune.
While the 2-dose regimen of inactivated hepatitis A vaccine is used in many countries, other countries may consider the inclusion of a single-dose inactivated hepatitis A vaccine in their immunization schedules.
Hepatitis A vaccination in the Americas
As of 2019, 17 of the 51 countries and territories in the Americas reported using the hepatitis A vaccine. Nine have introduced the hepatitis A vaccine as part of their national childhood immunization schedule.
PAHO's technical cooperation aims to:
- provide guidance to national immunization programs to conduct epidemiological and cost-effectiveness studies to inform a potential introduction of the hepatitis A vaccine for routine childhood vaccination.
- monitor hepatitis A vaccination policies and vaccination coverage in the Americas.
- provide access to affordable hepatitis A vaccines for countries through the Revolving Fund.