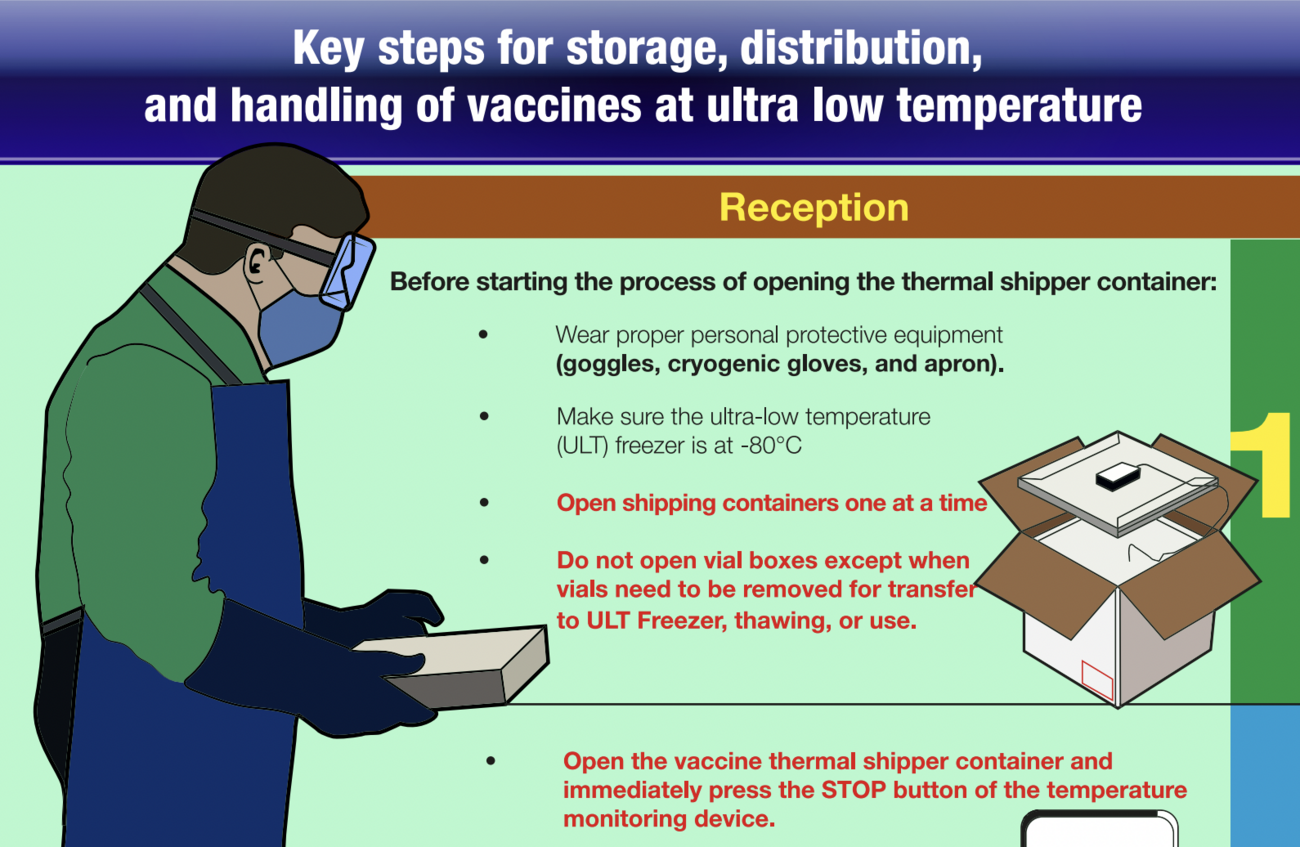
The strategic priority of the 2030 Immunization Agenda is the development of immunization programs for primary health care and universal health coverage.
The fulfilment of this goal requires, among other activities, to focus on monitoring the safety of vaccines and vaccination, in order to guarantee a supply chain of high quality vaccines and supplies. This is articulated in a service delivery system based on primary care.
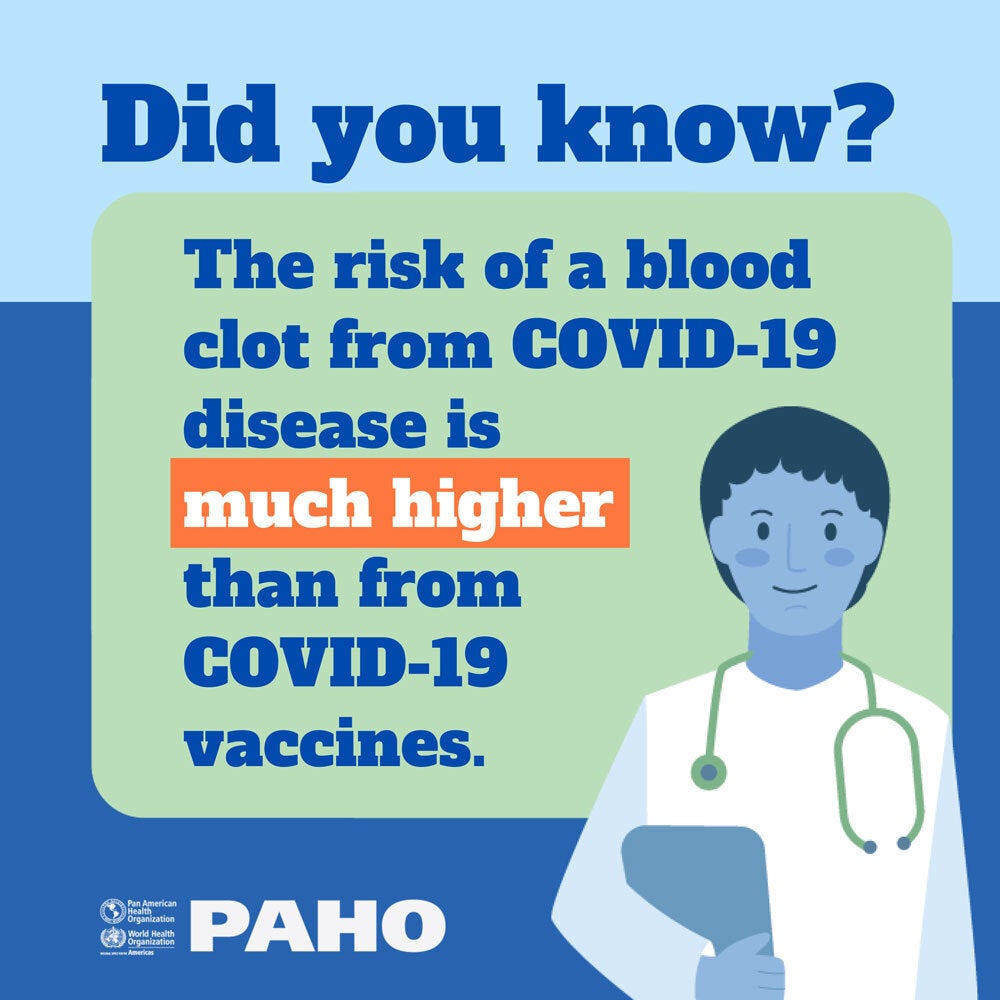
In the Region of the Americas, vaccines have reached high levels of acceptance and confidence in their effectiveness to reduce the frequency and impact of many infectious diseases, compared to other areas of the world. However, the public has expressed more doubts about the safety of vaccines than about their effectiveness.
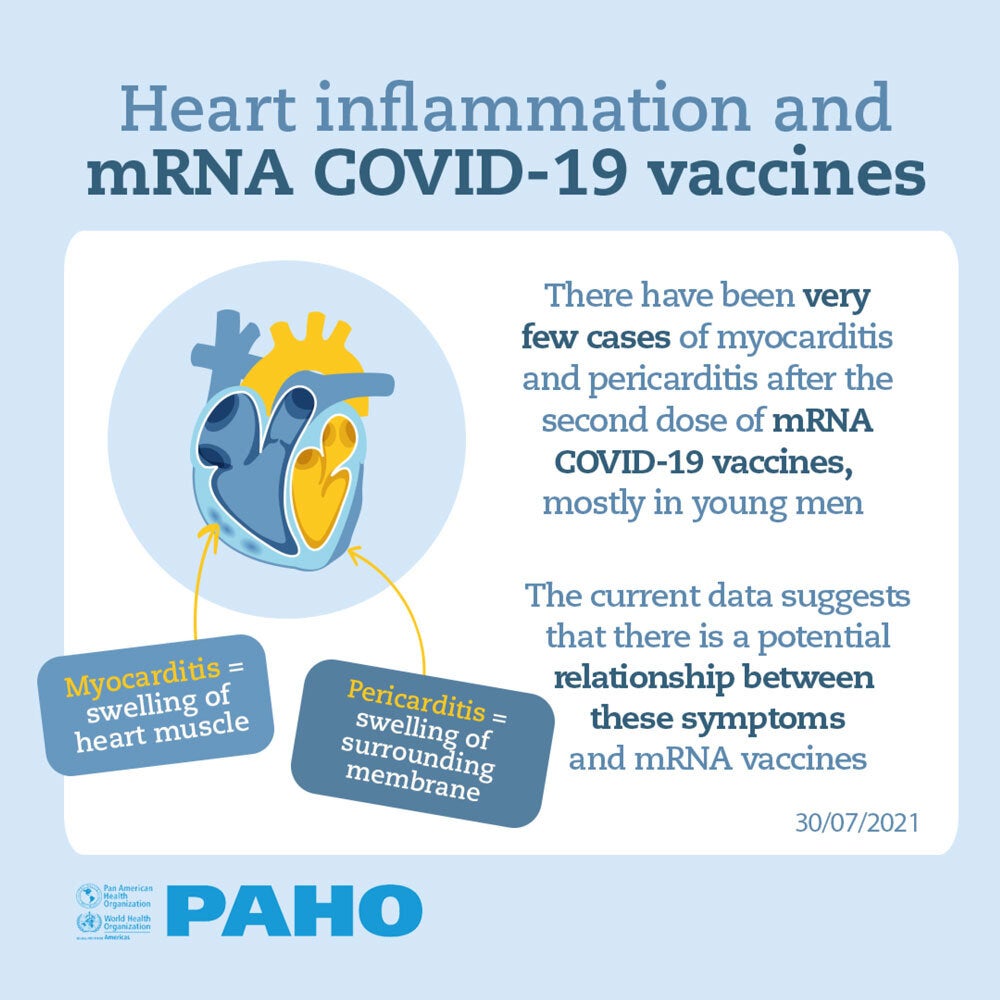
In the current context of the emergency due to the COVID-19 pandemic, in which the rapid deployment of new vaccines has been required and have had to go through emergency authorization schemes, an emphasis has been placed on activities that guarantee the Vaccine safety. These include the surveillance of Events Supposedly Attributable to Vaccination or Immunization (ESAVI).
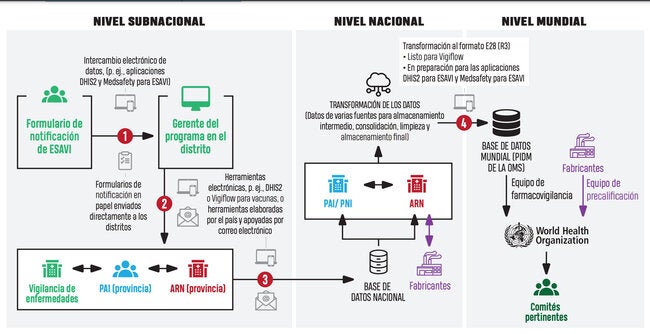
The safe vaccination system is made up of several components that must interact continuously and by activities carried out simultaneously by different institutions in all countries.
Vaccine Safety: An interinstitutional work
| All the countries in the Region of the Americas have national ESAVI surveillance systems, with different levels of maturity that depend on a variety of actors within the health system. | |
| In 17 countries of the Region, (48.6%), the national immunization program (NIP) is responsible for reporting ESAVI data; In nine countries (25.7%), ESAVIs are reported jointly between the NIP and the National Regulatory Authority (NRA) and in only three countries (8.6%) the responsibility is solely of the NRA. Six countries (17.1%) of the Region have designated an institution other than the NRA or NIP to report these data | |
| The monitoring of adverse events after the administration of vaccines generates useful information on rare and very rare adverse events, and on risks related to the quality and handling of vaccines. This information is used to minimize its recurrence and impact on the health of the population. | |
| Generating reliable information from ESAVIs allows countries to form objectives and provide timely response to questions from the public and national authorities about the use of vaccines. |