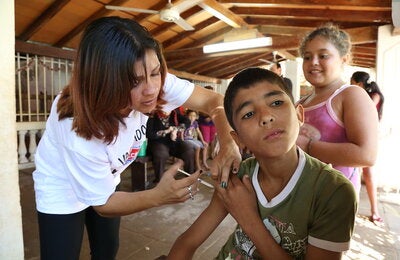
Bridgetown, Barbados, 1 November, 2021 (PAHO/WHO) - The Caribbean Regulatory System (CRS) of the Caribbean Public Health Agency (CARPHA), in collaboration with the Pan American Health Organization / World Health Organization (PAHO/WHO), launches a week-long social media campaign to promote the importance of reporting suspected side effects following vaccination to the CRS or to national reporting systems.
Taking place from 1–7 November 2021, the sixth annual #MedSafetyWeek social media campaign focusses on vaccines. Pharmaceutical agencies from 64 countries will be encouraging healthcare professionals, national immunisation programme staff, as well as patients, their carers and families, to report problems experienced with vaccines including COVID-19 shots.
Vaccines are among the most important advances in medicine saving millions of lives annually and have proven to be the best method to protect individuals against infectious diseases. However, as with all medicines, side effects can happen.
According to Mr. Dean Chambliss, PAHO Subregional Program Director, Caribbean, “This campaign comes at a critical moment when millions of people are vaccinated against COVID-19. Vaccine safety begins with the timely detection and reporting of adverse events following immunization. It is important to report suspected adverse events of vaccines and by extension all medicines. Each report counts but to have an impact, countries need to assess this information and share safety updates regularly with the international community.”
In August 2021, CARPHA launched its online reporting form for adverse events following immunization (AEFI) which can be used for any vaccine, including the novel COVID-19 vaccines.
Dr. Joy St. John, CARPHA Executive Director stated: “The reporting of an adverse effect following vaccination does not mean the event has been caused by the vaccine, or that the person who gave the vaccine made an error, but it is an important part of helping the national authorities to identify events that may need to be investigated further. The information from investigations and data analyses will assist Member States in identifying the types of reactions that persons are experiencing, and any additional monitoring or regulatory actions that may be needed.”
Reporting of side effects that last longer than three days, and those that cause persons to see a doctor is an important part of checking and ensuring that approved vaccines remain safe when used in larger populations. Health workers are encouraged to use the relevant vaccine product information from trusted sources, in order to guide patients, parents and caregivers.
For information about COVID-19 vaccines visit:
- WHO: COVID-19 Vaccine
- CARPHA’s Vaccine Information
- CARPHA-CRS page on the regional reporting system (VigiCarib)
- PAHO’s Vaccine Dashboard
Notes to Editor
- National medicines regulatory authorities from 64 countries across the globe and their stakeholders will be taking part in this international campaign led by Uppsala Monitoring Centre (UMC), the World Health Organization (WHO) Collaborating Centre for International Drug Monitoring. The campaign is supported by members of the Heads of Medicines Agencies (HMA) and the International Coalition of Medicines Regulatory Authorities (ICMRA). The #MedSafetyWeek 2021 project team consists of representatives from the following organisations working collaboratively: Medicines and Healthcare products Regulatory Agency (UK) as co-lead, Egypt Chapter of the International Society of Pharmacovigilance (ISoP Egypt), the Health Products Regulatory Authority (Ireland), and the Food and Drugs Authority (Ghana).
- Ministries of health in CARPHA Member States are responsible for protecting and improving the health of millions of people every day through the effective regulation of all vaccines and medicines in their countries by ensuring they work and are acceptably safe. Their work is underpinned by robust and fact-based judgements to ensure that the benefits justify any risks.
- CARPHA-CRS has a voluntary reporting system (VigiCarib) that supports the collection of reports of adverse events following immunization. Health care workers and patients may report to the VigiCarib using its online form, but patients (or other non-health professionals) are advised to consult a health professional for medical confirmation before submitting the report. National authorities will follow up with reporters according to national policies and procedures.
- Patients are advised to contact a healthcare professional if they are worried about their health.